
Concept explainers
a)
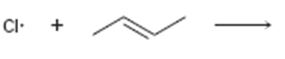
Interpretation:
Curved arrows are to be drawn to show the flow of electrons in the given reaction. The structure of carbon radical that is formed when the halogen radical add to the
Concept introduction:
In reactions involving free radicals homolytic cleavage of covalent bonds takes place. The free radicals produced in the initiation step reacts with the other reactant present in the propagation steps to yield new radicals.
To draw:
Curved arrows to show the flow of electrons in the given reaction and to show the structure of carbon radical that is formed when the halogen radical add to the alkenes.
b)
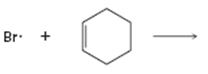
Interpretation:
Curved arrows are to be drawn to show the flow of electrons in the given reaction. The structure of carbon radical that is formed when the halogen radical add to the alkenes is to be drawn.
Concept introduction:
In reactions involving free radicals homolytic cleavage of covalent bonds takes place. The free radicals produced in the initiation step reacts with the other reactant present in the propagation steps to yield new radicals.
To draw:
Curved arrows to show the flow of electrons in the given reaction and to show the structure of carbon radical that is formed when the halogen radical add to the alkenes.
c)
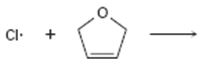
Interpretation:
Curved arrows are to be drawn to show the flow of electrons in the given reaction. The structure of carbon radical that is formed when the halogen radical add to the alkenes is to be drawn.
Concept introduction:
In reactions involving free radicals homolytic cleavage of covalent bonds takes place. The free radicals produced in the initiation step reacts with the other reactant present in the propagation steps to yield new radicals.
To draw:
Curved arrows to show the flow of electrons in the given reaction and to show the structure of carbon radical that is formed when the halogen radical add to the alkenes.
Trending nowThis is a popular solution!

Chapter 6 Solutions
Organic Chemistry
- Draw curved arrows to show the movement of the electrons that result in formation of the given product(s).arrow_forwardDraw the structure of the alkene that will give the alcohol in Figure 10 as the main product.arrow_forwardDraw the products of the following reactions. Use curved arrows to show where the pair of electrons starts and where it ends up.arrow_forward
- Use curved arrows to show the flow of electrons on the reactant side of each of the following reactions:arrow_forwardAdd one or more curved arrows to show the movement of electrons for each step in the substitution reaction. All charges and electrons are already drawn. Do not delete any pre‑drawn bonds, charges, or lone pairs. If you accidentally delete a vital part of the structure, click the undo button in the lower left.arrow_forwardUse curved arrows to draw the mechanism of this reaction below:arrow_forward
- Draw curly arrows to show electron flow in the last step of the aromatic bromination reaction below.arrow_forwardIn halogenation reactions, why are halogens added on opposite sides of the compound? because the reaction proceeds via free radical and the pi cloud holding the electron are directed away from each other because of the formation of a halonium ion which sterically blocks one side of the compound thus the other side is open for attack because the halogen should occupy both sides to accommodate the pi clouds None of the above Two equivalents of HCl are added to an alkyne, where do you expect to see the Cl atom? The two Cl atoms will be distributed between the two Carbon atoms involved in the triple bond The two Cl atoms forms a bond with the less substituted carbon Both Cl atoms attach to one carbon, which is the more substituted one A mixture of products are formedarrow_forwardWrite out the mechanism for this hydration of an alkene adding curved arrows to show how electrons reorient to make or break bonds or form new lone pairsarrow_forward
- Draw 2 different structures that contain carbocations that would undergo a methyl shift a ring expansion Use arrows to show how this would occurarrow_forwardComplete each reaction by putting the missing reagent or missing product.arrow_forwardfinish ONE of the reactions by filling in any starting materials, reagents, or products as needed.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
