
a)
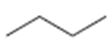
Interpretation:
The structures of different monochloro products, without considering their stereochemistry, obtainable by the radical chlorination of n-butane are to be drawn.
Concept introduction:
In radical chlorination reactions, if all the hydrogens present in the alkane are of same kind then it is possible to get a single monochloro product. If the alkane has hydrogens of different kinds then hydrogens belonging to all kinds will be substituted by chlorine resulting in a mixture of monochloro
To draw:
The structures of different monochloro products, without considering their stereochemistry, obtainable by the radical chlorination of n-butane.
b)
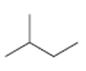
Interpretation:
The structures of different monochloro products, without considering their stereochemistry, obtainable by the radical chlorination of 2-methylbutane are to be drawn.
Concept introduction:
In radical chlorination reactions, if all the hydrogens present in the alkane are of same kind then it is possible to get a single monochloro product. If the alkane has hydrogens of different kinds then hydrogens belonging to all kinds will be substituted by chlorine resulting in a mixture of monochloro alkanes as product.
To draw:
The structures of different monochloro products, without considering their stereochemistry, obtainable by the radical chlorination of 2-methylbutane.
c)
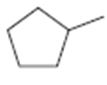
Interpretation:
The structures of different monochloro products, without considering their stereochemistry, obtainable by the radical chlorination of methylcyclopentane are to be drawn.
Concept introduction:
In radical chlorination reactions, if all the hydrogens present in the alkane are of same kind then it is possible to get a single monochloro product. If the alkane has hydrogens of different kinds then hydrogens belonging to all kinds will be substituted by chlorine resulting in a mixture of monochloro alkanes as product.
To draw:
The structures of different monochloro products, without considering their stereochemistry, obtainable by the radical chlorination of methylcyclopentane.
Trending nowThis is a popular solution!

Chapter 6 Solutions
Organic Chemistry
- Please explain Consider the reaction between methylcyclohexene and the hydroxyl radical.arrow_forwarda) How many monochlorination products can be obtained from the radical chlorination of methylcyclohexane? Disregard stereoisomers. b) Which product would be obtained in greatest yield? Briefly explain. c) How many monochlorination products would be obtained if all stereoisomers are included?arrow_forwardsee the attached question and out of these 3 comounds, which one is the best dienophile ?arrow_forward
- In theory, there are only three inequivalent hydrogens in this molecule that could be substituted by Br in a free radical bromination – circle them. Put an asterisk to mark the one most likely to be substituted first.arrow_forwardReasoning from Hammond’s postulate, predict the regioselectivity of radical fluorination relative to that of radical chlorination and bromination.arrow_forwardIf the following compound was reacted with Bromine and light under the same reaction conditions as the compounds from the experiment, approximately how long would it take to react? Explain your prediction. Draw the major product of the reaction .arrow_forward
- Draw the structure of the two products that would form during termination:- From the combination of two bromine radicals- From the combination of a bromine radical and an alkyl radicalThe outline of this product has already been provided for you to complete.Note: Make sure to include lone pairs in the structures.arrow_forwardFor each compound, predict the major product of free-radical bromination. Remember that bromination is highly selective, and only the most stable radical will be formed=methylcyclopentanearrow_forwardDraw and name all monochloro products you would expect to obtain from radicalchlorination of 2-methylpentane. Which, if any, are chiral?arrow_forward
- Fill the blank space. Compounds containing a phenol group may work as ANTIOXIDANTS to prevent free radical damage. This is accomplished when a free radical (or UV light) encounters a phenol group, turning the phenol group into a radical. However, contrary to typical radical behavior, the structure of the phenol radical can neutralize (or quench) the unpaired electron. Specifically, the phenol structure neutralizes (or quenches) the unpaired radical electron by doing the following: taking the electron and ---------. The correct name (or abbreviation) of an example compound containing a phenol group with antioxidant properties is: ---------.arrow_forwardwhat is the mechanism for this reaction and would be the structure of the product?arrow_forwardIndane can undergo free-radical chlorination at any of the alkyl positions onthe aliphatic ring. What instrumental technique would be most helpful for determining how many products are formed, and how many of those products are monochlorinated and how many are dichlorinated?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





