
Chemistry & Chemical Reactivity
9th Edition
ISBN: 9781133949640
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 7, Problem 60GQ
Answer the questions below concerning ground state electron configurations.
- (a) What element has the electron configuration [Ar]3d64s2?
- (b) What element has a 2+ ion with the configuration [Ar]3d5? Is the ion paramagnetic or diamagnetic?
- (c) Mow many unpaired electrons are in a Ni2+ ion?
- (d) The configuration for an element is given here.

What is the identity of the element? Is a sample of the element paramagnetic or diamagnetic? How many unpaired electrons does a 3− ion of this element have?
- (e) What element has the following electron configuration? Write a complete set of quantum numbers for electrons 1-3.
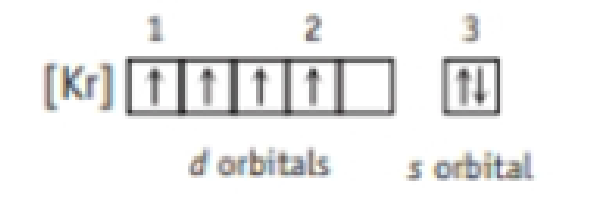
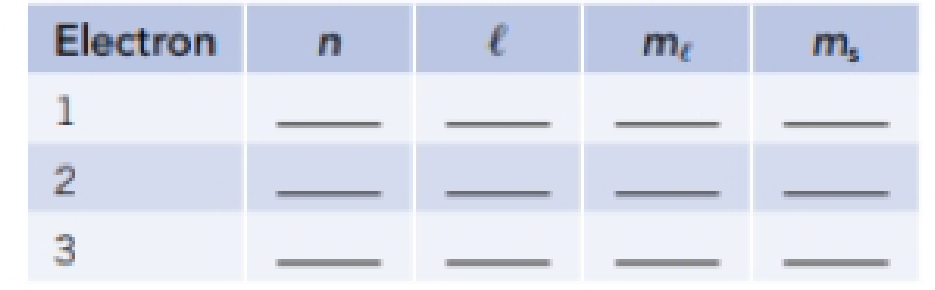
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Chemistry & Chemical Reactivity
Ch. 7.1 - How many electrons can be accommodated in the n =...Ch. 7.1 - Prob. 2RCCh. 7.2 - Based on the Aufbau principle and the n + rule,...Ch. 7.3 - (a) What element has the configuration...Ch. 7.3 - Write one possible set of quantum numbers for the...Ch. 7.3 - Using the periodic table and without looking at...Ch. 7.3 - 1. What is the electron configuration of selenium...Ch. 7.3 - 2. Based on electron configurations, which of the...Ch. 7.4 - Prob. 1CYUCh. 7.4 - Prob. 1RC
Ch. 7.4 - Prob. 2RCCh. 7.4 - Which of the following species is most...Ch. 7.5 - Without looking at the figures for the periodic...Ch. 7.5 - What is the trend in sizes of the ions K+, S2, and...Ch. 7.5 - Prob. 2RCCh. 7.6 - Give the electron configurations for iron and the...Ch. 7.6 - Prob. 2QCh. 7.6 - Prob. 3QCh. 7.6 - Prob. 4QCh. 7.6 - Prob. 1RCCh. 7.6 - Prob. 2RCCh. 7.6 - The most common oxidation state of a rare earth...Ch. 7.6 - Prob. 6QCh. 7.6 - Prob. 7QCh. 7.6 - Use the atomic radii of scandium, yttrium,...Ch. 7.6 - Prob. 9QCh. 7.6 - Prob. 10QCh. 7 - Write the electron configurations for P and CI...Ch. 7 - Write the electron configurations for Mg and Ar...Ch. 7 - Using spdf notation, write the electron...Ch. 7 - Using spdf notation, give the electron...Ch. 7 - Prob. 5PSCh. 7 - Prob. 6PSCh. 7 - Use noble gas and spdf notations to depict...Ch. 7 - The lanthanides, once called the rare earth...Ch. 7 - Prob. 9PSCh. 7 - Prob. 10PSCh. 7 - What is the maximum number of electrons that can...Ch. 7 - What is the maximum number of electrons that can...Ch. 7 - Depict the electron configuration for magnesium...Ch. 7 - Depict the electron configuration for phosphorus...Ch. 7 - Using an orbital box diagram and noble gas...Ch. 7 - Using an orbital box diagram and noble gas...Ch. 7 - Using orbital box diagrams, depict an electron...Ch. 7 - Prob. 18PSCh. 7 - Prob. 19PSCh. 7 - Using orbital box diagrams and noble gas notation,...Ch. 7 - Manganese is found as MnO2 in deep ocean deposits....Ch. 7 - One compound found in alkaline batteries is NiOOH,...Ch. 7 - Prob. 23PSCh. 7 - Arrange the following elements in order of...Ch. 7 - Prob. 25PSCh. 7 - Prob. 26PSCh. 7 - Which of the following groups of elements is...Ch. 7 - Arrange the following atoms in order of increasing...Ch. 7 - Compare the elements Na, Mg, O, and P. (a) Which...Ch. 7 - Compare the elements B. Al, C, and Si. (a) Which...Ch. 7 - Explain each answer briefly. (a) Place the...Ch. 7 - Explain each answer briefly. (a) Rank the...Ch. 7 - Identify the element that corresponds to each of...Ch. 7 - Identify the element that corresponds to each of...Ch. 7 - Explain why the photoelectron spectra of hydrogen...Ch. 7 - Sketch the major features (number of peaks and...Ch. 7 - These questions are not designated as to type or...Ch. 7 - The deep blue color of sapphires comes from the...Ch. 7 - Using an orbital box diagram and noble gas...Ch. 7 - Prob. 40GQCh. 7 - Prob. 41GQCh. 7 - Prob. 42GQCh. 7 - Which of the following is not an allowable set of...Ch. 7 - A possible excited state for the H atom has an...Ch. 7 - The magnet in the following photo is made from...Ch. 7 - Name the element corresponding to each...Ch. 7 - Arrange the following atoms in order of increasing...Ch. 7 - Prob. 48GQCh. 7 - Answer the questions below about the elements A...Ch. 7 - Answer (he following questions about the elements...Ch. 7 - Which of the following ions are unlikely to be...Ch. 7 - Prob. 52GQCh. 7 - Answer each of the following questions: (a) Of the...Ch. 7 - Prob. 54GQCh. 7 - Prob. 55GQCh. 7 - Two elements in the second transition series (Y...Ch. 7 - Prob. 57GQCh. 7 - The configuration of an element is given here. (a)...Ch. 7 - Answer the questions below about the elements A...Ch. 7 - Answer the questions below concerning ground state...Ch. 7 - Nickel(II) formate [Ni(HCO2)2] is widely used as a...Ch. 7 - Spinets are solids with the general formula M2+...Ch. 7 - The following questions use concepts from this and...Ch. 7 - Which ions in the following list are not likely to...Ch. 7 - Answer the following questions about first...Ch. 7 - The ionization of the hydrogen atom can be...Ch. 7 - Compare the configurations below with two...Ch. 7 - Prob. 68SCQCh. 7 - Write electron configurations to show the first...Ch. 7 - Prob. 70SCQCh. 7 - (a) Explain why the sizes of atoms change when...Ch. 7 - Which of the following elements has the greatest...Ch. 7 - Prob. 73SCQCh. 7 - Prob. 74SCQCh. 7 - The energies of the orbitals in many elements have...Ch. 7 - The ionization energies for the removal of the...Ch. 7 - Using your knowledge of the trends in element...Ch. 7 - Prob. 78SCQCh. 7 - Prob. 79SCQCh. 7 - Prob. 80SCQCh. 7 - Thionyl chloride. SOCl2, is an important...Ch. 7 - Prob. 82SCQCh. 7 - Slaters rules are a way to estimate the effective...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Does the information on alkali metals in Table 2-8 of the text confirm the general periodic trends in ionization energy and atomic radius? Explain.arrow_forwardIn what main group(s) of the periodic table do elements have the following number of half-filled p-orbitals in the outermost principal energy level? (a) 0 (b) 1(c) 2(d) 3arrow_forwardThe configuration of an element is given here. (a) What is the identity of the element? (b) In what group and period is the element found? (c) Is the element a nonmetal, a main group element, a transition metal, a lanthanide, or an actinide? (d) Is the element diamagnetic or paramagnetic? If paramagnetic, how many unpaired electrons are there? (e) Write a complete set of quantum numbers (n, , m, ms) for each of the valence electrons. (f) What is the configuration of the 2+ ion formed from this element? Is the ion diamagnetic or paramagnetic?arrow_forward
- The first ionization energy of helium is 2370kJmol1 , the highest for any element. (a) Define ionization energy and discuss why for helium it should be so high. (b) Which element would you expect to have the highest second ionization energy? Why? (c) Suppose that you wished to ionize some helium by shining electromagnetic radiation on it. What is the maximum wavelength you could use?arrow_forward6.82 A particular element has the following values for its first four ionization energies: 900, 1760, 14, 850, and 21,000 kJ/mol. Without consulting a list of ionization energy values, determine what group in the periodic table this element belongs in.arrow_forwardWrite an orbital diagram for the ground state of the zinc atom. Is the atomic substance diamagnetic or paramagnetic?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Periodic Properties of Elements | Chemistry | IIT-JEE | NEET | CBSE | Misostudy; Author: Misostudy;https://www.youtube.com/watch?v=L26rRWz4_AI;License: Standard YouTube License, CC-BY
Periodic Trends: Electronegativity, Ionization Energy, Atomic Radius - TUTOR HOTLINE; Author: Melissa Maribel;https://www.youtube.com/watch?v=0h8q1GIQ-H4;License: Standard YouTube License, CC-BY