
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
6th Edition
ISBN: 9781305687875
Author: Gilbert
Publisher: CENGAGE LEARNING - CONSIGNMENT
expand_more
expand_more
format_list_bulleted
Question
Chapter 7.3, Problem 4E
Interpretation Introduction
Interpretation:
The stepwise mechanism using curved arrow to show the electron’s flow for the below isomerization needs to be determined.
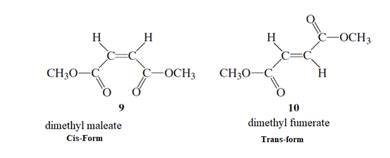
Concept Introduction :
The isomerism procedure includes the isomers. It is known as the molecules that contain the similar molecular formula but they generally own a dissimilar atom’s arrangement in space. Below are the two kinds:
- Constitutional isomers: They are known as the compounds within which the several atoms are linked or attached in a dissimilar manner. They are also known as the structural isomers:
- Stereoisomers: These are the compounds that vary on the basis of positioning in space. Below are two types:
- Enantiomers: These are the compounds that are generally a mirror image of one another and these mirror images are also known as non-superimposable.
- Diastereomers: These are the compounds that are not the mirror images of one another. Also, these compounds are also non-superimposable. They are of two kinds: cis and trans:
- Cis-isomers are the isomers that generally have atoms that are concerned with the similar side of a bond.
- Instead, the trans-isomers are the compounds which contain atoms concerned with opposite sides of a bond.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
I need help on writing all of the
Materials used in the expirment
Experiment 8 Preparation of Cyclohexanone by Hypochlorite Oxidation
How would you recrystallize a mixture of Benzoic acid and 2-Naphthol? Discuss the choice of solvent and recrystallization process. (Hint. Look up the solubility of these two compounds in different solvents)
Please create a flow chart for this experiment. ( the images are steps 1 through five, the steps 6-9 are in the description below.)
Steps 6-9:
—>
Cautiously add concentrated hydrochloric acid dropwise to Flask 1 until the contents are acidic to litmus and then cool the flask in ice.
7) Decant the ether from Flask 3 into a tared flask, taking care to leave all of the drying agent behind. Wash the drying agent with additional ether to ensure complete transfer of the product. If decantation is difficult then remove the drying agent by gravity filtration. Put a boiling stick in the flask, and evaporate the ether in the hood.
8) Isolate the compound in Flask 2 by vacuum filtration
on a Hirsch funnel, and wash it on the filter with a small
quantity of ice water.
9) Isolate, weight the product in Flask 1 by suction filtration.
Chapter 7 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
Ch. 7.2 - Prob. 1ECh. 7.2 - Prob. 2ECh. 7.2 - Prob. 3ECh. 7.2 - Prob. 4ECh. 7.2 - Prob. 5ECh. 7.2 - Prob. 6ECh. 7.2 - Prob. 7ECh. 7.2 - Prob. 8ECh. 7.2 - Prob. 9ECh. 7.2 - Prob. 10E
Ch. 7.3 - Prob. 1ECh. 7.3 - Prob. 2ECh. 7.3 - Prob. 3ECh. 7.3 - Prob. 4ECh. 7.3 - Prob. 5ECh. 7.3 - Prob. 6ECh. 7.3 - Prob. 7ECh. 7.3 - Prob. 8ECh. 7.4 - Prob. 1ECh. 7.4 - Prob. 2ECh. 7.4 - Prob. 3ECh. 7.4 - Prob. 4ECh. 7.4 - Prob. 5ECh. 7.4 - Prob. 6ECh. 7.4 - Prob. 7ECh. 7.4 - Prob. 8ECh. 7.4 - Prob. 9ECh. 7.4 - Prob. 10ECh. 7.4 - Prob. 11ECh. 7.4 - Prob. 12ECh. 7.4 - Prob. 13ECh. 7.6 - Prob. 1ECh. 7.6 - Prob. 2ECh. 7.6 - Prob. 3ECh. 7.6 - Prob. 4ECh. 7.6 - Prob. 5ECh. 7.6 - Prob. 6ECh. 7.6 - Prob. 7ECh. 7.6 - Prob. 8ECh. 7.6 - Prob. 9ECh. 7.6 - Prob. 10E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- This experiment is the Separation of a mixture of 3-nitroaniline, Benzoic acid, and naphthalene using an acid-base separation extraction technique What properties of the three compounds in this experiment allow you to use the above technique to separate them?Why are you asked to use multiple portions of the solvent at the extraction steps?Why are you asked not to discard any solvents until you have obtained your products?arrow_forwardAfter the 3rd addition of bromine, the orange color persisted, even after swirling for 5 minutes. What does this indicate? At this point, some drops of 1-hexene were added, and the color immediately faded away to give ivory-colored crystals. Explain this observation, and show the reaction of the 1-hexene with the appropriate chemical.arrow_forwardWhat is the reason behind the portion-by-portion evaporation of DCM in the extraction of eugenol?arrow_forward
- Based on the readings and lecture on emulsions, and the procedures for this lab answer the following question. What is the dispersed phase in the simple vinaigrette? Air Oil Water Eggarrow_forwardGive the reason for collecting the distillate above 190°C in the flow chart.arrow_forwardReaction with Bromine a. In another test tube, put 10 drops of cyclohexene and 5 drops of bromine and cover the tube with a clean cork b. Shake the tube to mix the contents c. Let it stand for a minute or two to complete the reaction d. Test the gases in the test tube with a piece of blue litmus paper moistened with distilled water and held with tweezers Observations____________________________________ ______________________________________________________________________________________________arrow_forward
- Why does the eugenol move from the water layer to the dichloromethane layer during extraction?arrow_forwardWrite the reaction involved in Ferrox Test. a. What is the species responsible? b. Why is phenol negative in Ferrox Test? Based on the theoretical result, what is the order of reactivity of primary, secondary, and tertiary alcohols in the Lucas Test? a. Lucas Reagent contains ZnCl2 in HCl. What is the role of ZnCl2?arrow_forward1. You have been given a mixture of toluene,aniline and benzoic acid dissolved in chloroform.explain in detail with the aid of a flow diagram and reaction schemes,how you would separate the mixture into pure componentsarrow_forward
- 5-bromoacetylsalicylic acid melts at 60 °C and is inert and almost insolube in water at room temperature. Why can’t water be used as recrystallizing solvent for this compound?arrow_forward1. Briefly discuss the roles of (1) placing tea bags in boiling water, and (b) addition of anhydrous sodium sulfate in the isolation of the alkaloid. 2. Some procedures include the addition of sodium carbonate before liquid-liquid extraction. What is the purpose of this step? 3. Give at least three characteristics of dichloromethane that makes it a good extracting solvent for the alkaloid. 4. Why is it necessary to remove a stopper from a separatory funnel when the liquid is being drained from it through a stopcock? 5. What are emulsions? Why do they form during extractions? How is the formation of an emulsion minimized? How are emulsions removed?arrow_forwardWrite the reaction involved in Ferrox Test. a. What is the species responsible? b. Why is phenol negative in Ferrox Test? Based on the theoretical result, what is the order of reactivity of primary, secondary, and tertiary alcohols in the Lucas Test? a. Lucas Reagent contains ZnCl2 in HCl. What is the role of ZnCl2? What reagents are used in the esterification of Alcohols and Phenols? a. Write the reaction involved in Primary Alcohol (Ethanol) and Acetyl Chloride b. Write the reaction involved in Phenol and Acetyl Chloride What is the purpose of the Chromic acid test? a. What are the reagents used? b. Write oxidation reaction of Primary Alcohols and Secondary Alcoholsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
Seven Name Reactions in One - Palladium Catalysed Reaction (047 - 053); Author: Rasayan Academy - Jagriti Sharma;https://www.youtube.com/watch?v=5HEKTpDFkqI;License: Standard YouTube License, CC-BY