
Concept explainers
(a)
Interpretation:
Whether the given pair of 1, 2-dibromocyclohexane is enantiomers or diastereomer needs to be determined. This also needs to be indicated whether each pair haveidentical or different physical properties.
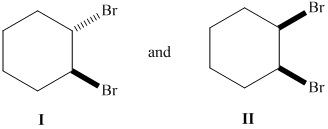
Concept Introduction :
The stereoisomers having different configurations at all stereocenters are known as enantiomers of each other and if there is a different configuration at only one stereocenter but same at other and both isomers are the non-mirror image of each other, they will be diastereomers.
(b)
Interpretation:
Whether the given pair of 1, 2-dibromocyclohexane is enantiomers or diastereomer needs to be determined. This also needs to be indicated whether each pair haveidentical or different physical properties.
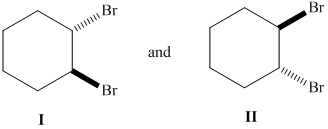
Concept Introduction :
The stereoisomers having different configurations at all stereocenters are known as enantiomers of each other and if there is a different configuration at only one stereocenter but same at other and both isomers are the non-mirror image of each other, they will be diastereomers.
(c)
Interpretation:
Whether the given pair of 1, 2-dibromocyclohexane is enantiomers or diastereomer needs to be determined. This also needs to be indicated whether each pair haveidentical or different physical properties.
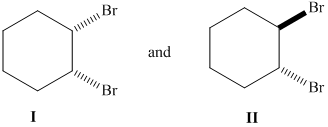
Concept Introduction :
The stereoisomers having different configurations at all stereocenters are known as enantiomers of each other and if there is a different configuration at only one stereocenter but same at other and both isomers are the non-mirror image of each other, they will be diastereomers.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
- Following is a planar hexagon representation of L-fucose, a sugar component of the determinants of the A, B, O blood group typing. For more on this system of blood typing, see Chemical Connections: A, B, AB, and O Blood Group Substances in Chapter 25. (a) Draw the alternative chair conformations of L-fucose. (b) Which of them is more stable? Explain.arrow_forwardKetones react with alcohols to yield products called acetals. Why does the all-cis isomer of 4-tert-butyl-1,3-cyclohexanediol react readily with acetone and an acid catalyst to form an acetal, but other stereoisomers do not react? In formulating your answer, draw the more stable chair conformations of all four stereoisomers and the product acetal for each one.arrow_forwardFor the molecule given, prioritize each substituent on the stereogenic carbon, and assign absolute stereochemistry.arrow_forward
- Identify the stereogenic carbon in (S)- and (R)-limonene, rank the substituents around it and rationalize the assignment of their stereochemical configurations.arrow_forwardThe following are representations of two forms of glucose. The six-membered ring is known to exist in a chair conformation in each form. Draw clear representations of the most stable conformation of each. Are they two different conformations of the same molecule, or are they stereoisomers that cannot be interconverted by rotation about single bonds? Which substituents (if any) occupy axial sites?arrow_forwardAny enantiomers, stereocenters, diastereomers or meso compounds for dibutylcyclohexane?arrow_forward
- Define the degree of unsaturation ?arrow_forwardGive the stereochemical relationships between each pair of structures. Examples are same compound, structural isomers, enantiomers, diastereomers. Which pairs could you (theoretically) separate by distillation or recrystallization?*refer to the photo belowarrow_forwardDraw all stereoisomers for the following molecules; indicate the stereochemical relationships for all possible pairsarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

