
Concept explainers
Interpretation:
The atomic orbital overlap and MO energy diagrams for
Concept introduction:
According to
The molecular orbitals are formed by overlapping of atomic orbitals of adjacent atoms. The numbers of molecular orbitals formed are equal to the number of atomic orbitals overlap. The two atomic orbitals, on mixing along bonding axes, form two molecular orbitals, one is
Answer to Problem D.15YT
The atomic orbital overlap and MO energy diagrams for
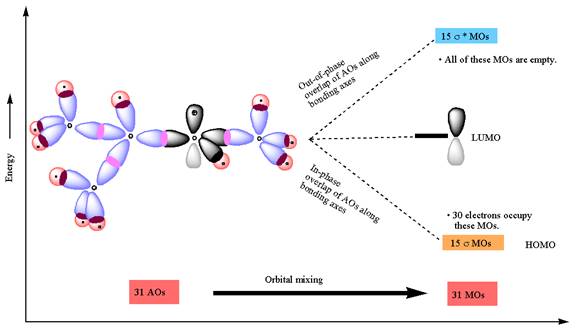
The constructed MO energy diagrams for
Explanation of Solution
In
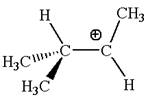
In forming the
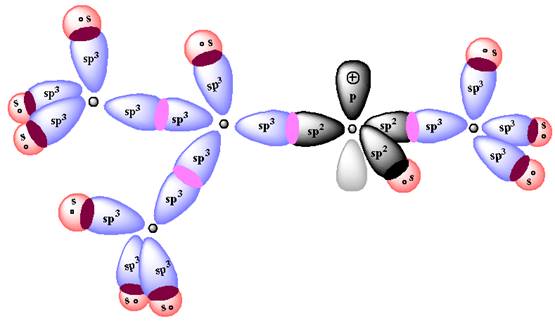
According to Aufbau Principle, the
The energy diagram for the formation of
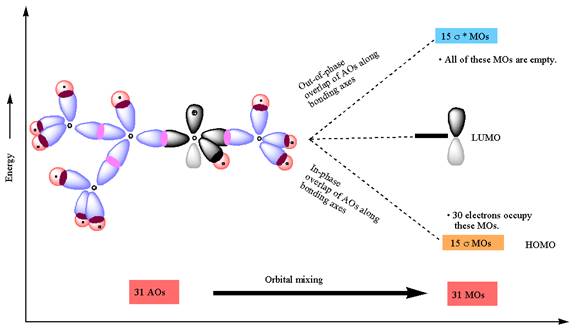
The Figure
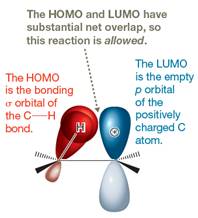
The constructed MO energy diagrams for
The energy diagram for the formation of
Want to see more full solutions like this?
Chapter D Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

