
Concept explainers
(a)
Interpretation:
The structure of the molecule is to be drawn and the IUPAC name is to be provided that corresponds to the given trivial name.
Concept introduction:
Carboxylic acids are the compounds containing
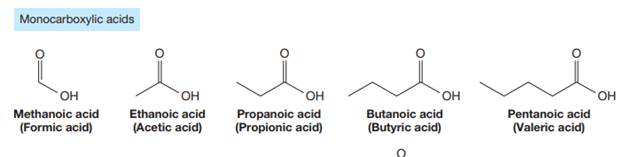
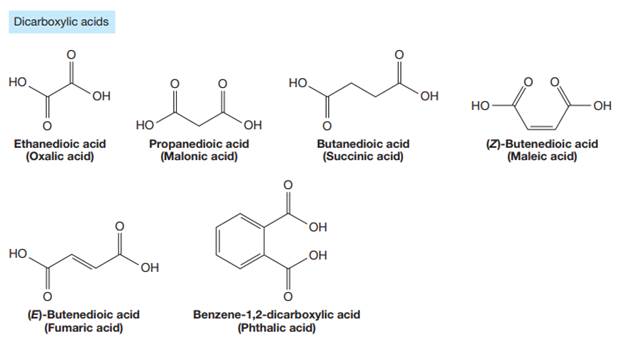
The carbonyl carbon atom is always numbered as C1 and the numbering continues. The substituents, if any, attached to the carbon chain are names according to the alphabetical order and the lowest locator number.
The trivial names of carboxylic acids are in the general form alkionic acid, while the IUPAC names are in the general form alkanoic acid. The suffix ‘ionic’ is replaced by ‘noic’ in the IUPAC name.
Answer to Problem F.12P
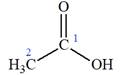
The structure of the molecule that corresponds to the given trivial name
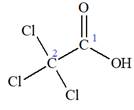
The IUPAC name of
Explanation of Solution
The given IUPAC name is
The portion ‘
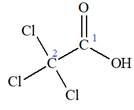
The IUPAC name of
The structure of the compound is drawn and the IUPAC name of the compound is written that corresponds to the given trivial name.
(b)
Interpretation:
The structure of the molecule is to be drawn and the IUPAC name is to be provided that corresponds to the given trivial name.
Concept introduction:
Carboxylic acids are the compounds containing
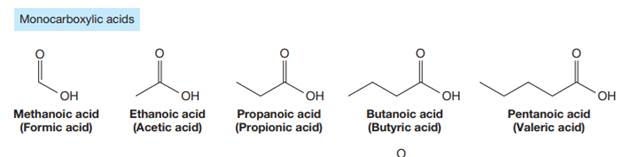
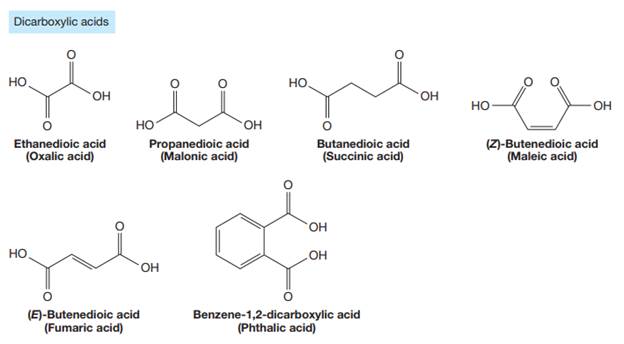
The carbonyl carbon atom is always numbered as C1 and the numbering continues. The substituents, if any, attached to the carbon chain are names according to the alphabetical order and the lowest locator number.
The trivial names of carboxylic acids are in the general form alkionic acid, while the IUPAC names are in the general form alkanoic acid. The suffix ‘ionic’ is replaced by ‘noic’ in the IUPAC name.
Answer to Problem F.12P
The structure of the molecule that corresponds to the given trivial name
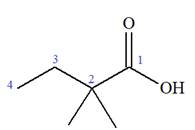
The IUPAC name of
Explanation of Solution
The given IUPAC name is
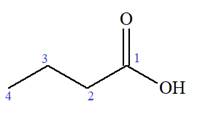
The prefix ‘2, 2-dimethyl’ indicates that the methyl group is attached at C2, and C2 carbon atoms of the butyric acid. The structure of
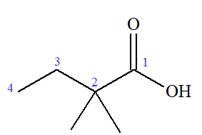
The trivial names of carboxylic acids are in the general form alkionic acid while the IUPAC names are in the general form alkanoic acid. The suffix ‘ionic’ is replaced by ‘noic’ in the IUPAC name.
The butyric acid is the trivial name, its IUPAC name is butanoic acid. Thus, the IUPAC name of
The structure of the compound is drawn and the IUPAC name of the compound is written that corresponds to the given trivial name.
(c)
Interpretation:
The structure of the molecule is to be drawn and the IUPAC name is to be provided that corresponds to the given trivial name.
Concept introduction:
Carboxylic acids are the compounds containing
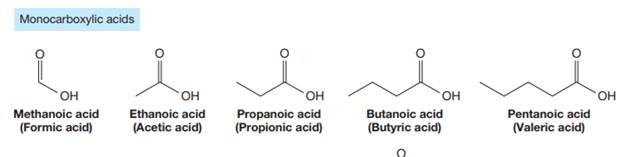
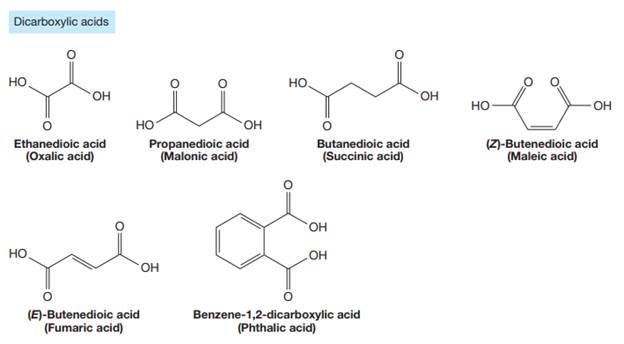
The carbonyl carbon atom is always numbered as C1 and the numbering continues. The substituents, if any, attached to the carbon chain are names according to the alphabetical order and the lowest locator number.
The trivial names of carboxylic acids are in the general form alkionic acid, while the IUPAC names are in the general form alkanoic acid. The suffix ‘ionic’ is replaced by ‘noic’ in the IUPAC name.
Answer to Problem F.12P
The structure of the molecule that corresponds to the given trivial name
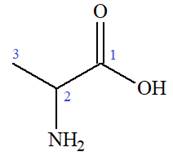
The IUPAC name of
Explanation of Solution
The given IUPAC name is
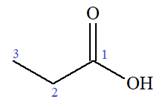
The prefix ‘2-amino’ indicates that

The trivial names of carboxylic acids are in the general form alkionic acid while the IUPAC names are in the general form alkanoic acid. The suffix ‘ionic’ is replaced by ‘noic’ in the IUPAC name.
The propionic acid is the trivial name; its IUPAC name is propanoic acid. Thus, the IUPAC name of
The structure of the compound is drawn and the IUPAC name of the compound is written that corresponds to the given trivial name.
(d)
Interpretation:
The structure of the molecule is to be drawn and the IUPAC name is to be provided that corresponds to the given trivial name.
Concept introduction:
Carboxylic acids are the compounds containing


The carbonyl carbon atom is always numbered as C1 and the numbering continues. The substituents, if any, attached to the carbon chain are names according to the alphabetical order and the lowest locator number.
The trivial names of carboxylic acids are in the general form alkionic acid, while the IUPAC names are in the general form alkanoic acid. The suffix ‘ionic’ is replaced by ‘noic’ in the IUPAC name.
Answer to Problem F.12P
The structure of the molecule that corresponds to the given trivial name
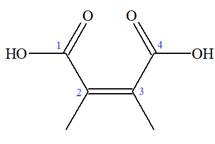
The IUPAC name of
Explanation of Solution
The given IUPAC name is
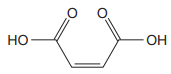
Note the stereochemistry about the doubly bonded carbon atoms. The two high-priority groups are on the same side of the double bond making it (Z) stereoisomer.
The prefix
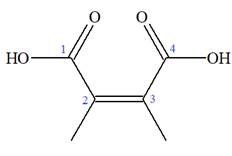
As the longest carbon chain has four atoms, the root name for carboxylic acid is
The structure of the compound is drawn and the IUPAC name of the compound is written that corresponds to the given trivial name.
(e)
Interpretation:
The structure of the molecule is to be drawn and the IUPAC name is to be provided that corresponds to the given trivial name.
Concept introduction:
Carboxylic acids are the compounds containing
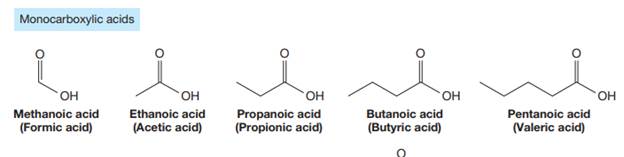
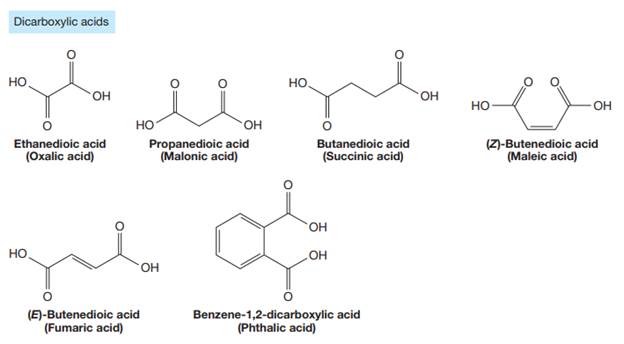
The carbonyl carbon atom is always numbered as C1 and the numbering continues. The substituents, if any, attached to the carbon chain are names according to the alphabetical order and the lowest locator number.
The trivial names of carboxylic acids are in the general form alkionic acid, while the IUPAC names are in the general form alkanoic acid. The suffix ‘ionic’ is replaced by ‘noic’ in the IUPAC name.
Answer to Problem F.12P
The structure of the molecule that corresponds to the given trivial name
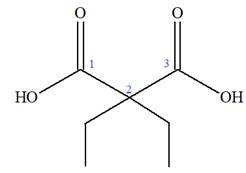
The IUPAC name of
Explanation of Solution
The given IUPAC name is
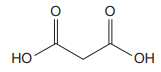
The prefix ‘diethyl’ in the name suggests that an ethyl group is attached at C2 carbon atom of the root. The locator numbers 2 and 2 are not included in the name because there is only one carbon atom to which any substituent could get attached. Thus, the complete structure of

As the longest carbon chain has three atoms, the root name for this dicarboxylic acid is
The structure of the compound is drawn and the IUPAC name of the compound is written that corresponds to the given trivial name.
Want to see more full solutions like this?
Chapter F Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


