HO HC CH3 compound A With a flask of certified primary standard of pure compound A, the researcher carried out the absorbance measurement (U.V. at 298 nm) of 6 different concentrations, using methanol and a 10 mm quartz cuvette and obtained the following results: C(ug/mL) Absorbância 0,2962 0,3943 0,4924 0,5905 4 9. 0,6886 0,7867 Knowing that at 298nm the peak of highest intensity occurs in the U.V. indicate what kind of transition is this probably referring to? With the data in the table determines the molar extinction coefficient of compound A? The absorbance reading of the injectable showed a value of 0.4436, what is the real value of concentration of this injectable? The absorbance reading of the tablet showed a value of 0.4826, which is the real value of mass of the asset contained in the tablet knowing that it was dissolved completely in 100mL of methanol? 00
HO HC CH3 compound A With a flask of certified primary standard of pure compound A, the researcher carried out the absorbance measurement (U.V. at 298 nm) of 6 different concentrations, using methanol and a 10 mm quartz cuvette and obtained the following results: C(ug/mL) Absorbância 0,2962 0,3943 0,4924 0,5905 4 9. 0,6886 0,7867 Knowing that at 298nm the peak of highest intensity occurs in the U.V. indicate what kind of transition is this probably referring to? With the data in the table determines the molar extinction coefficient of compound A? The absorbance reading of the injectable showed a value of 0.4436, what is the real value of concentration of this injectable? The absorbance reading of the tablet showed a value of 0.4826, which is the real value of mass of the asset contained in the tablet knowing that it was dissolved completely in 100mL of methanol? 00
Chapter28: Atomic Spectroscopy
Section: Chapter Questions
Problem 28.13QAP
Related questions
Question
100%
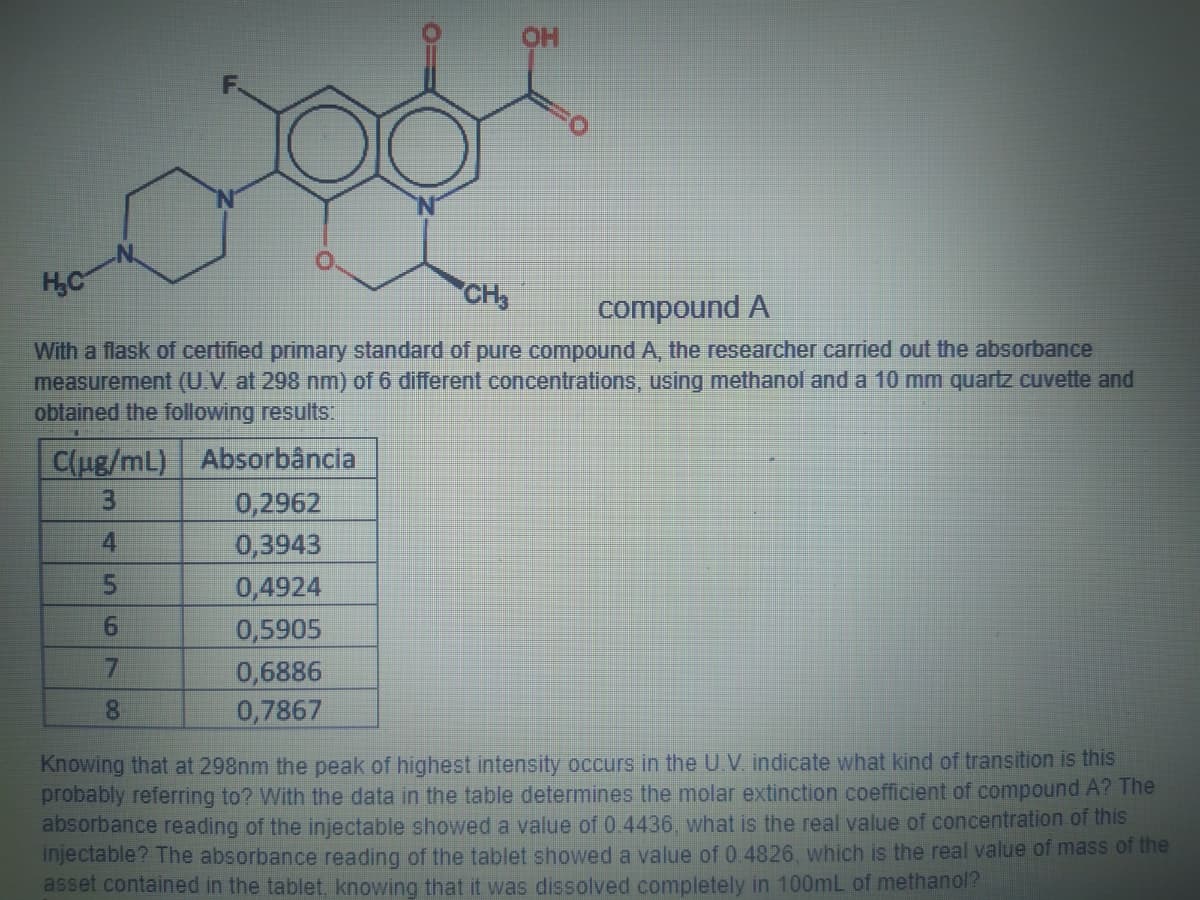
Transcribed Image Text:OH
HC
CH
compound A
With a flask of certified primary standard of pure compound A, the researcher carried out the absorbance
measurement (U.V. at 298 nm) of 6 different concentrations, using methanol and a 10 mm quartz cuvette and
obtained the following results:
C(ug/mL) Absorbância
0,2962
0,3943
3.
4.
0,4924
0,5905
0,6886
0,7867
7.
8.
Knowing that at 298nm the peak of highest intensity occurs in the U.V. indicate what kind of transition is this
probably referring to? With the data in the table determines the molar extinction coefficient of compound A? The
absorbance reading of the injectable showed a value of 0.4436, what is the real value of concentration of this
injectable? The absorbance reading of the tablet showed a value of 0.4826, which is the real value of mass of the
asset contained in the tablet knowing that it was dissolved completely in 100mL of methanol?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning