
Concept explainers
(a)
Interpretation:
It is to be determined whether and how[DK1] the given ether can be produced from a
Concept introduction:
The Williamson ether synthesis is the most convenient method for an ether synthesis. In this synthesis, an
Answer to Problem 10.16P
The given ether can be produced successfully from Williamson ether synthesis as below:
Explanation of Solution
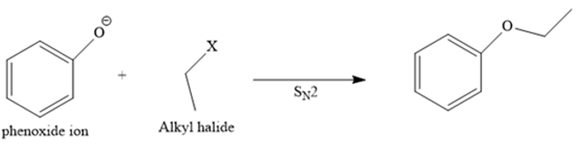
The structure of the given ether is
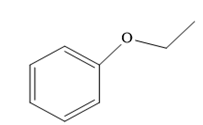
In this ether, one of the R groups is a phenyl ring, and the other is an ethyl group.
So, there are two routes to produce the desired ether by Williamson ether synthesis. Route one is discussed below.
Route I:
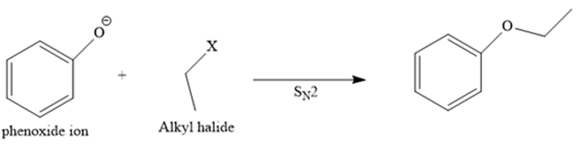
This is a feasible synthesis because the phenoxide ion is a good nucleophile, and the halide group attached on the primary carbon atom (primary alkyl halide) is a good substrate for a
The second possible route is discussed below.
Route II:
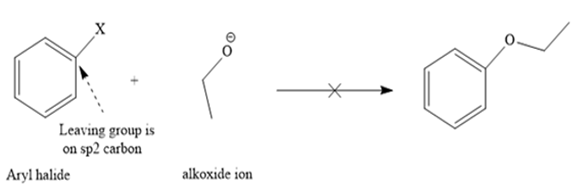
In this method, the halide group is on sp2 hybridized carbon, which is not acceptable for an
As Williamson ether synthesis is an
(b)
Interpretation:
It is to be determined whether and how[DK2] the given ether can be produced from a Williamson ether synthesis. And if there are two feasible syntheses for the given ether, it is to be determined which one is more preferable.
Concept introduction:
The Williamson ether synthesis is the most convenient method for an ether synthesis. In this synthesis, an alkyl halide
Answer to Problem 10.16P
The given ether cannot be synthesized by Williamson ether synthesis.
Explanation of Solution
The structure of the given ether is
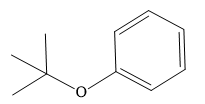
In this ether, one of the R groups is a phenyl ring, and the other is a tertiary butyl group. Those two groups would be the potential alkyl halides for a Williamson ether synthesis reaction. Route I is shown below:
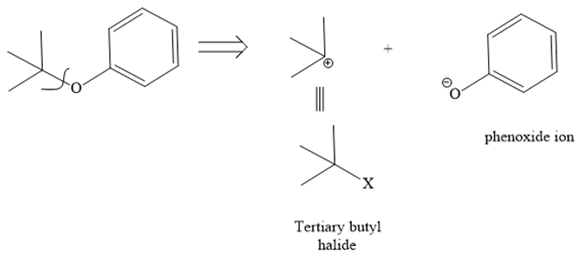
The retrosynthesis suggests that the given ether can be synthesized from a tertiary butyl halide as a substrate and a phenoxide ion as a nucleophile. But the alkyl halide has a leaving group on the tertiary carbon, so it will not follow an
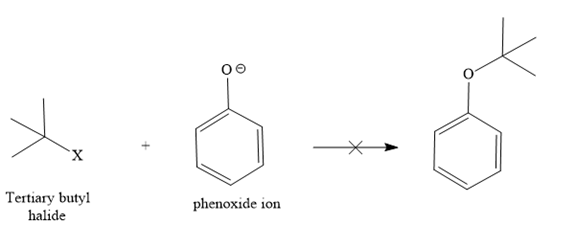
Instead, it shows an E1 reaction with the phenoxide ion because the phenoxide ion acts as a base instead of the nucleophile due to bulkiness.
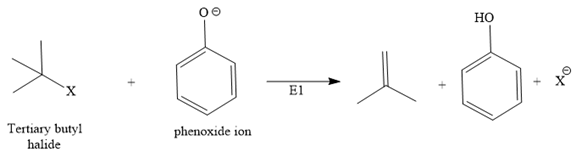
The second route is not acceptable because the positive charge comes on [DK4] the carbon atom of a phenyl ring, which is already electron-rich and sp2, which is not good for the
Route II:
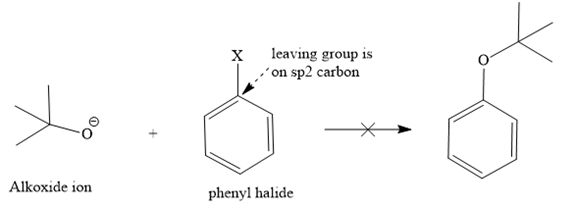
Since both routes do not give the desired ether as a product via
As Williamson ether synthesis is an
(c)
Interpretation:
It is to be determined whether and how[DK5] the given ether can be produced from a Williamson ether synthesis. And if there are two feasible syntheses for the given ether, it is to be determined which one is more preferable.
Concept introduction:
The Williamson ether synthesis is the most convenient method for an ether synthesis. In this synthesis, an alkyl halide
Answer to Problem 10.16P
The given ether can be successfully produced from a Williamson ether synthesis via two routes as below:
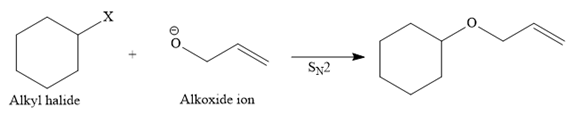
Route I:
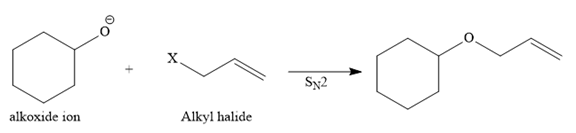
Route II:
The first route is more preferable as it makes use of a primary alkyl halide as a substrate.
Explanation of Solution
The given ether is
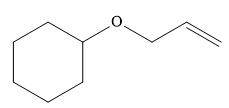
One R group in the given ether is cyclohexane while the other is an allyl group. Both of these R groups can be potentially used as substrates in the Williamson ether synthesis. Route I is shown below:
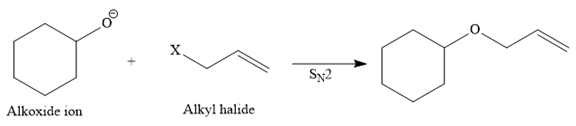
In this route, the leaving group (halogen atom, X) is on the primary carbon, and an alkoxide ion is also a good nucleophile, so the reaction can proceed through
The other route for the synthesis of the given ether is shown below:
Route II:

In this route, the leaving group (halogen atom, X) is on the secondary carbon, and an alkoxide ion is also a good nucleophile, so the reaction can proceed through
Note that both routes are feasible for the given ether synthesis, but the substrate of both routes is different. In the first route, the substrate (alkyl halide) has a leaving group on primary carbon while in the second route it is on the secondary carbon. Since an
As Williamson ether synthesis is an
Want to see more full solutions like this?
Chapter 10 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Predict/draw the major product of the reaction shown in the picture:arrow_forwardPredict the major organic product for each of the reactions that completes the transformationarrow_forwardDraw the complete, detailed E1 mechanism for each of the following reactions, and show all resonance structures, where applicable.arrow_forward
- Determine the major product of each reaction in Problem and draw the complete, detailed mechanism. Pay attention to stereochemistry where appropriate.arrow_forwardDraw the complete, detailed mechanism for the following reaction.arrow_forwardDraw a mechanism to account for the reaction shown here, which scrambles the isotopic labelingarrow_forward
- Please answer question below, be very deatiled and draw out the mechanism reaction with arrowsarrow_forwardperform williamson etherification synthesis on the following molecule. Show all steps of the mechanismarrow_forwardOrganic Chemistry Problem. Please help with figuring out what steps to take to obtain the product of the reaction shown. Thank youarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
