
Concept explainers
(a)
Interpretation:
The complete and detailed mechanism is to be drawn for the given reaction.
Concept introduction:
The SN1 is an abbreviation for unimolecular nucleophilic substitution reaction. These reactions are two-step reactions. The first step is the formation of carbocation intermediate, followed by the nucleophilic attack, which is a second step. Due to the formation of a planar carbocation, the incoming nucleophile can attack the carbocation from two sides, either from the front of the plane or from the back side of the plane.
This leads to give the mixture of stereoisomers. When secondary and tertiary alcohols are treated with strong concentrated acids, it follows SN1 and E1 mechanism.
Answer to Problem 10.1P
The complete and detailed mechanism for given reaction is as follows:
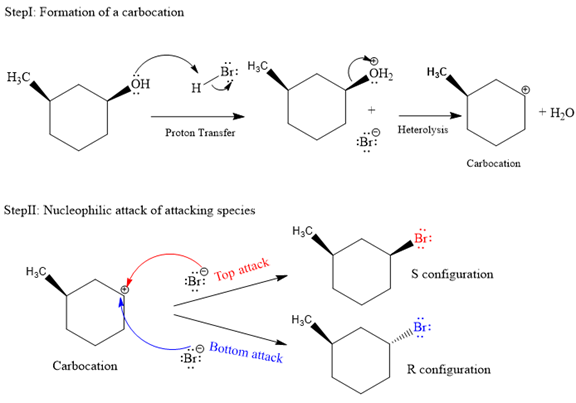
Explanation of Solution
The given reaction is as follows:
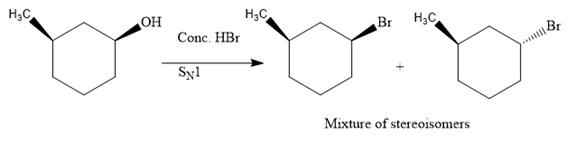
The hydroxyl (
The departure of water is a slow step that leads to formation of carbocation, and thus it is the rate-determining step. Due to the formation of planar carbocation intermediate, the incoming nucleophile can attack from either sides, either the top (from above the plane) or bottom (from below of plane). The bromide ion formed in the proton transfer step is the attacking species in the second step of the mechanism. In the second step, the bromide ion (
Both top and bottom attack of nucleophile are possible leads to formation of the mixture of isomers, as shown below:
Due to the formation of carbocation intermediate in SN1 reaction, a mixture of stereoisomer is produced.
(b)
Interpretation:
The complete and detailed mechanism is to be drawn for the given reaction.
Concept introduction:
The SN1 is an abbreviation for unimolecular nucleophilic substitution reaction. These reactions are two-step reactions. The first step is the formation of carbocation intermediate, followed by the nucleophilic attack of attacking species, which is a second step. Due to the formation of a planar carbocation, the incoming nucleophile can attack the carbocation from two sides, either from the front of the plane or from the back side of the plane.
This leads to give the mixture of stereoisomers. When secondary and tertiary alcohols are treated with strong concentrated acids, it follows SN1 and E1 mechanism.
Answer to Problem 10.1P
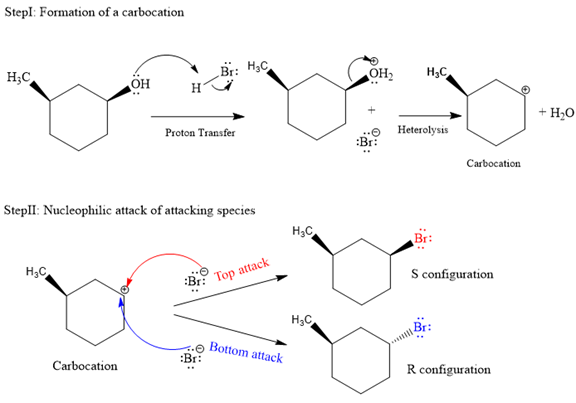
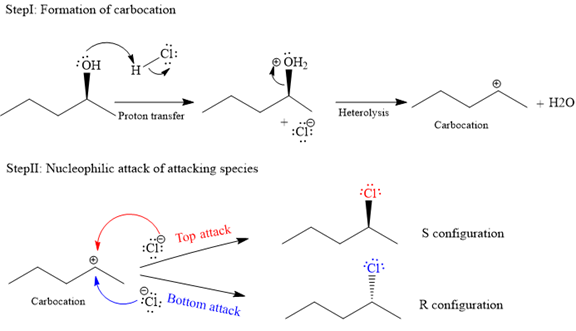
The complete and detailed mechanism for given reaction is as follows:
Explanation of Solution
The given reaction is as follows:
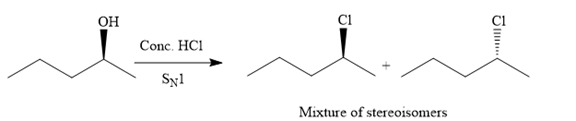
The hydroxyl (
Both top and bottom attack of nucleophile are possible leads to formation of the mixture of isomers, as shown below:

Due to the formation of carbocation intermediate in SN1 reaction, a mixture of stereoisomer is produced.
Want to see more full solutions like this?
Chapter 10 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Draw the product, state the reaction type and draw out the key steps of the mechanism.arrow_forwardThe reaction shown here is a halosulfonation, which is a useful variation of the sulfonation reaction. Draw the complete mechanism for this reaction.arrow_forwardPlease draw arrow-pushing reaction mechanism and identify number of electrons involved here. This is an electrolytic reactionarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
