
(a)
Interpretation:
The normal boiling point of dichlorodimethylsilane has to be determined
Concept Introduction:
Boiling point of a liquid: The temperature at which external pressure and vapour pressure of the liquid become same.
Normal boiling point: When the external pressure is
(a)
Answer to Problem 39IL
The normal boiling point of dichlorodimethylsilane is
The temperatures at which liquid have a vapour pressures of
The molar enthalpy of vaporization of is
Explanation of Solution
The normal boiling point of dichlorodimethylsilane is calculated
Given:
Normal boiling point is the temperature when the external pressure is
From the given data it is clear that the temperature at which the pressure is
Thus the normal boiling point of dichlorodimethylsilane is
(b)
Interpretation:
The graph of
Concept Introduction:
Clausius-Clapeyron equation:
From this relationship we can calculate the molar enthalpy of vaporization by knowing the corresponding temperature and pressure values.
If we have pressures at two different temperatures, then enthalpy of vaporization can be calculated by
(b)
Answer to Problem 39IL
Using the given data we can plot the graph of
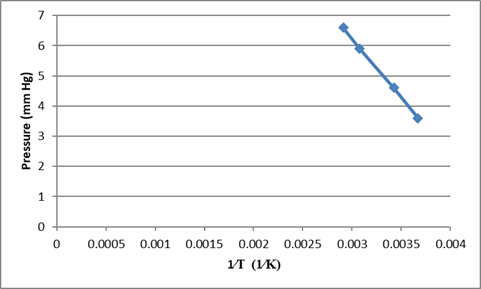
The temperature at which liquid has a vapour pressure of
The temperature at which liquid has a vapour pressure
Explanation of Solution
The temperatures at which liquid have a vapour pressures of
Given:
The values of
Using the given data we can plot the graph of
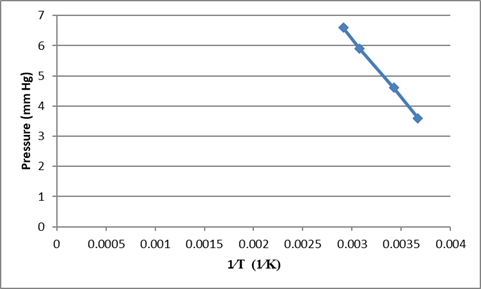
From the slope of the graph we can find the value of
Using the equation for the straight line in the plot
C, the constant value can be calculated by substituting any one of the value of pressure and temperature from the table given in the equation.
Substituting the values
From this equation we can calculate the temperature at which the pressures are
When the pressure is
The temperature at which the pressures is
When the pressure is
The temperature at which the pressures is
(c)
Interpretation:
The molar enthalpy of vaporization has to be explained.
Concept Introduction:
Clausius-Clapeyron equation:
From this relationship we can calculate the molar enthalpy of vaporization by knowing the corresponding temperature and pressure values.
If we have pressures at two different temperatures, then enthalpy of vaporization can be calculated by
Boiling point of a liquid: The temperature at which external pressure and vapour pressure of the liquid become same.
Normal boiling point: When the external pressure is
Molar enthalpy of vaporization: The energy required to convert liquid to gas of 1mol of a substance is called molar enthalpy of vaporization
(c)
Answer to Problem 39IL
The molar enthalpy of vaporization of is
Explanation of Solution
Given:
The molar enthalpy of vaporization using the given data is calculated.
Substituting the values
The molar enthalpy of vaporization using the given data is
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry & Chemical Reactivity
- Which compound or element has the lowest boiling point: Cl 2, KI, or LiF?arrow_forwardPlot lnP vs 1/T (in K!) and determine the enthalpy of vaporization of nitric acid at its boiling temperature. The vapor pressure of nitric acid is as follows: T(C): 0 20 50 70 90 100 P(Torr): 14.4 47.9 208 467 937 1282arrow_forwardThe vapor pressure, p, of nitric acid varies with temperature as follows: Ꝋ/oC 0 20 40 50 70 80 90 100 p/kPa 1.92 6.38 17.7 27.7 62.3 89.3 124.9 170.9 What are. The normal boiling point and The enthalpy of vaporization of nitric acid?arrow_forward
- Naphthalene undergoes sublimation: C10H8(s) ⇄ C10H8(g). In a closed container at 600.0 K, the equilibrium vapor pressure is 0.35 torr. What is the value of Kc?arrow_forwardWhich has the greatest vapour pressure at 25°C? SiO2 CO2 H2Oarrow_forwardWhat is boiling point of bromine when external pressure is 75kPa?arrow_forward
- Could you measure the triple point of water by measuring the temperature in a vessel in which water vapor, liquid water, and ice are in equilibrium under 1 atm of air? Explain.arrow_forwardAt which temperature is the vapor pressure of ethanol equal to 80. kPa?arrow_forwardDraw and explain pressure-density phase diagram for CO2.arrow_forward
- A recently developed variation of the Mond process carries out the first stepat higher pressures and at a temperature of 150°C. Estimate the maximumpressure of Ni(CO)4(g) that can be attained before the gas will liquefy atthis temperature (that is, calculate the vapor pressure of Ni(CO)4(e) at150°C).arrow_forwardIf the enthalpy of vaporization for water is 44.0kJ/mol and the vapor pressure of water at 56oC is 123.8mmHg, what is the vapor pressureat 95oC?arrow_forwardThe vapour pressure of hexane between -10°C and +90°C fits the expression log(p/Torr) = 7.724 – 1655/(T/K). Calculate the enthalpy of vaporization.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


