
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11, Problem 8PS
Which of the following compounds would be expected to form intermolecular hydrogen bonds in the liquid state?
- (a) H2Se
- (b) HCO2H (formic acid)
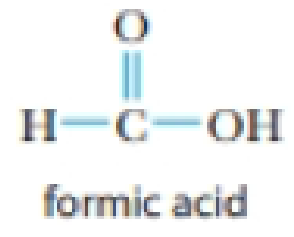
- (c) HI
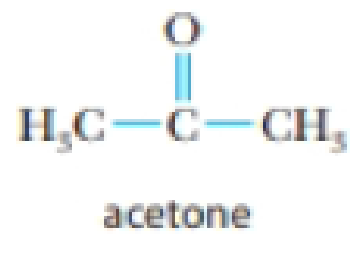
- (d) acetone, (CH3)2CO
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 11 Solutions
Chemistry & Chemical Reactivity
Ch. 11.2 - Which should have the more negative hydration...Ch. 11.3 - Using structural formulas, describe the hydrogen...Ch. 11.4 - Prob. 11.3CYUCh. 11.5 - Prob. 11.4CYUCh. 11.6 - The molar enthalpy of vaporization of methanol,...Ch. 11.6 - Prob. 11.6CYUCh. 11.6 - Prob. 1.1ACPCh. 11.6 - Prob. 1.2ACPCh. 11.6 - Prob. 2.1ACPCh. 11.6 - Prob. 2.2ACP
Ch. 11 - Prob. 1PSCh. 11 - Intermolecular forces: What type of forces must be...Ch. 11 - Prob. 3PSCh. 11 - Prob. 4PSCh. 11 - Considering intermolecular forces in the pure...Ch. 11 - Considering intermolecular forces in the pure...Ch. 11 - Prob. 7PSCh. 11 - Which of the following compounds would be expected...Ch. 11 - Prob. 9PSCh. 11 - When salts of Mg2+, Na+, and Cs+ are placed in...Ch. 11 - Prob. 11PSCh. 11 - The enthalpy of vaporization of liquid mercury is...Ch. 11 - Answer the following questions using Figure 11.12:...Ch. 11 - Answer the following questions using Figure 11.12:...Ch. 11 - Prob. 15PSCh. 11 - Refer to Figure 11.12 to answer these questions:...Ch. 11 - Which member of each of the following pairs of...Ch. 11 - Place the following four compounds in order of...Ch. 11 - Prob. 19PSCh. 11 - You are comparing three different substances, A,...Ch. 11 - Equilibrium vapor pressures of benzene, C6H6, at...Ch. 11 - Prob. 22PSCh. 11 - Can carbon monoxide (Tc = 132.9 K; Pc = 34.5 atm...Ch. 11 - Methane (CH4) cannot be liquefied at room...Ch. 11 - What is surface tension? Give an example...Ch. 11 - What factors affect the viscosity of a substance?...Ch. 11 - If a piece of filter paper (an absorbent paper...Ch. 11 - When water is placed in a buret it forms a concave...Ch. 11 - Prob. 29GQCh. 11 - What types of intermolecular forces are important...Ch. 11 - Which of the following salts, Li2SO4 or Cs2SO4, is...Ch. 11 - Prob. 32GQCh. 11 - Prob. 33GQCh. 11 - Prob. 34GQCh. 11 - Rank the following compounds in order of...Ch. 11 - Prob. 36GQCh. 11 - Prob. 37GQCh. 11 - The following data are the equilibrium vapor...Ch. 11 - Prob. 39ILCh. 11 - A hand boiler can be purchased in toy stores or at...Ch. 11 - Prob. 41ILCh. 11 - Prob. 42ILCh. 11 - Acetone, CH3COCH3, is a common laboratory solvent....Ch. 11 - Cooking oil floats on top of water. From this...Ch. 11 - Liquid ethylene glycol, HOCH2CH2OH, is one of the...Ch. 11 - Liquid methanol, CH3OH, is placed in a glass tube....Ch. 11 - Account for these facts: (a) Although ethanol...Ch. 11 - Prob. 48SCQCh. 11 - Prob. 49SCQCh. 11 - Prob. 50SCQCh. 11 - Prob. 51SCQCh. 11 - Prob. 52SCQCh. 11 - A fluorocarbon, CF4, has a critical temperature of...Ch. 11 - Prob. 55SCQCh. 11 - List four properties of liquids that are directly...Ch. 11 - List the following ions in order of hydration...Ch. 11 - Prob. 59SCQCh. 11 - An 8.82-g sample of Br2 is placed in an evacuated...Ch. 11 - Polarizability is defined as the extent to which...Ch. 11 - Prob. 62SCQCh. 11 - A pressure cooker (a kitchen appliance) is a pot...Ch. 11 - Vapor pressures of NH3() at several temperatures...Ch. 11 - Prob. 65SCQCh. 11 - Prob. 66SCQCh. 11 - Prob. 67SCQ
Additional Science Textbook Solutions
Find more solutions based on key concepts
Draw a Lewis structure for each covalent molecule. a. HBr b. CH3F c. H2O2 d. N2H4 e. C2H6 f. CH2Cl2
Principles of General, Organic, Biological Chemistry
1. What did each of the following scientists contribute to our knowledge of the atom?
a. William Crookes
b. E...
Chemistry For Changing Times (14th Edition)
covered a synthesis of alkynes by a double dehydrohalogenation of dihalides. A student tried to convert trans-2...
Organic Chemistry (9th Edition)
The chapter sections to review are shown in parentheses at the end of each problem. A "chemical-free” shampoo i...
Basic Chemistry
22.102 Write the structures of the cis and tram isomers, if any, for the following compounds:
Chemistry: The Molecular Nature of Matter
Q2. Which statement best defines chemistry?
a. The science that studies solvents, drugs, and insecticides
b. Th...
Introductory Chemistry (6th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The compounds ethanol (C2H5OH) and dimethyl ether (CH3OCH3) have the same molecular formula. Which is expected to have the higher surface tension? Why?arrow_forwardSilane SiH4, phosphine (PH3), and hydrogen sulfide (H2S) melt at 185 C, 133 C, and 85 C, respectively. What does this suggest about the polar character and intermolecular attractions of the three compounds?arrow_forwardIn which of the following processes is it necessary to break covalent bonds as opposed to simply overcoming intermolecular forces? (a) Decomposing HCI to H2 and Cl2 (b) Dissolving NaCl in water (c) Freezing ethyl alcohol (d) Subliming iodinearrow_forward
- 8.40 Which of the following compounds would be expected to form intermolecular hydrogen bonds in the liquid state? (a) CH3OCH3(dimethyl ether), (b) CH4(c) HF, (d) CH3CO2H(accetic acid), (e) Br2(f) CH3OH(methanol)arrow_forward8.48 Why must the vapor pressure of a substance be measured only after dynamic equilibrium is established?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning
Viscosity, Cohesive and Adhesive Forces, Surface Tension, and Capillary Action; Author: Professor Dave Explains;https://www.youtube.com/watch?v=P_jQ1B9UwpU;License: Standard YouTube License, CC-BY