
(a)
Interpretation: Full electronic configuration, orbital box diagram and the noble gas shorthand configuration for the given elements is to be given.
Concept Introduction: Electronic configuration is the arrangement of electrons in the increasing order of energy in the sub-shells of an atom.
(a)
Explanation of Solution
Full electronic configuration, orbital box diagram and the noble gas shorthand configuration for an element with atomic number 19 are
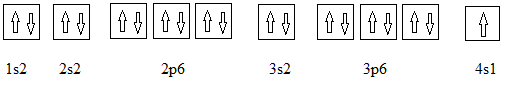
and
(b)
Interpretation: Full electronic configuration, orbital box diagram and the noble gas shorthand configuration for the given elements is to be given.
Concept Introduction: Electronic configuration is the arrangement of electrons in the increasing order of energy in the sub-shells of an atom. Atomic number is equal to the number of electrons present in the atom of an element.
(b)
Answer to Problem 62A
Full electronic configuration, orbital box diagram and the noble gas shorthand configuration for an element with atomic number 22 are
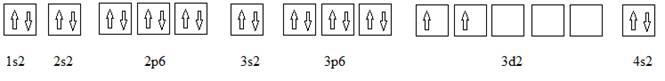
and
Explanation of Solution
The filling of electrons in the sub-shells follows Hund’s rule according to which lower energy orbitals are filled first followed by higher energy orbitals and Pauli’s exclusion principle according to which only two electrons will be present in an orbital and that too with opposite spin.
(c)
Interpretation: Full electronic configuration, orbital box diagram and the noble gas shorthand configuration for the given elements is to be given.
Concept Introduction: Electronic configuration is the arrangement of electrons in the increasing order of energy in the sub-shells of an atom. Atomic number is equal to the number of electrons present in the atom of an element.
(c)
Answer to Problem 62A
Full electronic configuration, orbital box diagram and the noble gas shorthand configuration for an element with atomic number 19 are
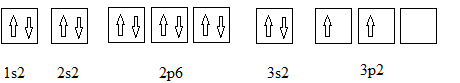
and
Explanation of Solution
The filling of electrons in the sub-shells follows Hund’s rule according to which lower energy orbitals are filled first followed by higher energy orbitals and Pauli’s exclusion principle according to which only two electrons will be present in an orbital and that too with opposite spin.
(d)
Interpretation: Full electronic configuration, orbital box diagram and the noble gas shorthand configuration for the given elements is to be given.
Concept Introduction: Electronic configuration is the arrangement of electrons in the increasing order of energy in the sub-shells of an atom. Atomic number is equal to the number of electrons present in the atom of an element.
(d)
Answer to Problem 62A
Full electronic configuration, orbital box diagram and the noble gas shorthand configuration for an element with atomic number 26 are
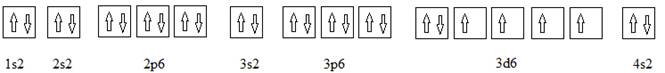
and
Explanation of Solution
The filling of electrons in the sub-shells follows Hund’s rule according to which lower energy orbitals are filled first followed by higher energy orbitals and Pauli’s exclusion principle according to which only two electrons will be present in an orbital and that too with opposite spin.
(e)
Interpretation: Full electronic configuration, orbital box diagram and the noble gas shorthand configuration for the given elements is to be given.
Concept Introduction: Electronic configuration is the arrangement of electrons in the increasing order of energy in the sub-shells of an atom. Atomic number is equal to the number of electrons present in the atom of an element.
(e)
Answer to Problem 62A
Full electronic configuration, orbital box diagram and the noble gas shorthand configuration for an element with atomic number 30 are
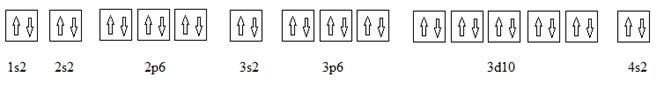
and
Explanation of Solution
The filling of electrons in the sub-shells follows Hund’s rule according to which lower energy orbitals are filled first followed by higher energy orbitals and Pauli’s exclusion principle according to which only two electrons will be present in an orbital and that too with opposite spin.
Chapter 11 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





