
Concept explainers
a.
Interpretation:
Product formed when below alcohol is oxidized in presence of K2Cr2O7 has to be determined
Concept introduction:
Oxidation of alcohol:
Duringoxidation of alcohol, the number of
b.
Interpretation:
Product formed when below alcohol is oxidized in presence of K2Cr2O7 has to be determined.
Concept introduction:
Refer to part ‘a.’.
c.
Interpretation:
Product formed when below alcohol is oxidized in presence of K2Cr2O7 has to be determined.
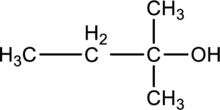
Concept introduction:
Refer to part ‘a.’.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
- What carbonyl compound is needed to make each alcohol by a reduction reaction? What carbonyl compound is needed to make each alcohol by a reduction reaction?arrow_forwardGive the IUPAC name for each alcohol но сн CH,CHCCH,CH,CH3 (CH)½CHCH,CHCH,CH3 b. a. CH,CH,CH,OH HO. с. CH,CH,CH,CH, Draw the products formed when each alcohol is dehydrated with H2SO4. Use the Zaitsev rule to predict the major product when a mixture forms. OH он b. -CHCH,CH3 а. Он с. CH3CHCH2CH,CH;CH3arrow_forward18. Ketone reduction Dicyclohexyl ketone Reduce the ketone. 1. NaBH4, ethanol 2. H3O+ H OH Dicyclohexylmethanol (88%) (a 2° alcohol)arrow_forward
- 4. What alcohol is formed when each compound is treated with NaBH4 in MEOH? a. NaBH MeOH H3CH,CH,C H. NABH4 b. MEOH C. NaBH MEOH Section: Row: 12 Column: 4 Words: 114 O Spell Check O Local backup on Page Num: 1 Page: 1/4 1/1 MacBook Pro 411 F6 F4 esc FI F2 & %23 24 3 4. 6. R T tab Caarrow_forwardDraw the products formed when each alcohol is dehydrated with H 2SO 4. Use the Zaitsev rule to predict the major product when a mixture forms.arrow_forwardWhat alcohol is formed when each carbonyl compound is treated with H 2 and a Pd catalyst?arrow_forward
- Classify each alcohol as to 1º, 2º, or 3º 1. 2. CH3CH2CH(CH3)CH2OHCH3 3. CH2CH2CHOHCH3arrow_forward2. Complete the following reactions for the preparation of alcohols. Draw the structure of the product. Name the reactant and the product. a) CHy CH=CH-CHy + HyO Hydration of alkenes b) Cat. CHy-CHy + H2 drogenation carbonyl pups d)arrow_forwardClassify each alcohol as 1 °, 2 °, or 3 °arrow_forward
- Classify each alcohol as 1 °, 2 °, or 3 °arrow_forwardWhat alcohol is formed when the compound is treated with H2 and a Pd catalyst?arrow_forward3. Complete the following intramolecular dehydration reactions for alcohols. Draw the structure of the product. Name the reactant and the product. a) Cy-C-CH-oH 18UC b) CHy CH-Cy-CHy 180C OH c) 180c 4. Complete the following intermolecular dehydration reactions for alcohols. Draw the structure of the product. Name the reactant and the product. a) CHy-CH-OH b) Ho SわふわSわ 5. Complete the following oxidation reactions for alcohols. Draw the structure of the product. Name the reactant and identify the type of compound formed in the product. a) hparrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning 
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning




