
Organic Chemistry
7th Edition
ISBN: 9780321803078
Author: Bruice, Paula Yurkanis
Publisher: Pearson College Div
expand_more
expand_more
format_list_bulleted
Question
Chapter 13, Problem 41P
Interpretation Introduction
Interpretation:
The change in the degree of regioselectivity compared to the ratio of relative rates found in problem 44 has to be given.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Chlorine radical forms a tertiary radical five times faster than a primary radical and it forms a secondary radical 3.8 times faster than a primary radical.
Relative rates of alkyl radical formation by a chlorine radical at 125 °C.
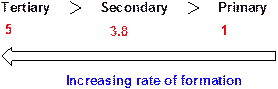
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
As in the free-radical halogenation of alkanes, chlorination of alkylbenzenes is less selective than bromination. Given the relative rates per hydrogen for hydrogen atom abstraction from 1-phenylbutane by chlorine for the elementary step shown, calculate the percentage of 1-chloro-1-phenylbutane in the C10H13Cl product.
R. Atkinson (J. Phys. Chem. Ref. Data 26, 215 (1997)) has reviewed a large set of rate constants relevant to the atmospheric chemistry of volatile organic compounds. The recommended rate constant for the bimolecular reaction of O2 with an alkyl radical R at 298 K is 4.7 × 109 dm3 mol−1 s−1for R = C2H5 and 8.4 × 109 dm3 mol−1 s−1 for R = cyclohexyl. Assuming no energy barrier, compute the steric factor, P, for each reaction. Hint: Obtain collision diameters from collision cross-sections of similar molecules in the Resource section.
The second-order rate constants for the reaction of oxygen atoms with aromatic hydrocarbons have been measured (R. Atkinson and J.N. Pitts, J. Phys. Chem. 79, 295 (1975)). In the reaction with benzene the rate constants are 1.44 × 107 dm3 mol−1 s−1 at 300.3 K, 3.03 × 107 dm3 mol−1 s−1 at 341.2 K, and 6.9 × 107 dm3 mol−1 s−1 at 392.2 K. Find the frequency factor and activation energy of the reaction.
Chapter 13 Solutions
Organic Chemistry
Ch. 13.2 - Prob. 1PCh. 13.2 - Write the steps for formation of...Ch. 13.3 - Prob. 3PCh. 13.4 - Prob. 4PCh. 13.5 - Prob. 7PCh. 13.5 - a. Would chlorination or bromination produce a...Ch. 13.5 - Prob. 10PCh. 13.6 - Prob. 11PCh. 13.7 - Prob. 12PCh. 13.7 - Prob. 13P
Ch. 13.8 - Prob. 14PCh. 13.8 - Draw the stereoisomers of the major...Ch. 13.9 - a. How many stereoisomers are formed from the...Ch. 13.9 - Prob. 17PCh. 13.9 - Prob. 19PCh. 13.9 - Prob. 20PCh. 13.9 - Prob. 21PCh. 13.10 - Prob. 22PCh. 13.11 - How many atoms share the unpaired electrons in...Ch. 13.11 - Prob. 24PCh. 13 - Prob. 25PCh. 13 - Prob. 26PCh. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 29PCh. 13 - Prob. 30PCh. 13 - Prob. 31PCh. 13 - Prob. 32PCh. 13 - Prob. 33PCh. 13 - Prob. 34PCh. 13 - Prob. 35PCh. 13 - Starting with cyclohexane, how could the following...Ch. 13 - a. Propose a mechanism for the following reaction:...Ch. 13 - What stereoisomers are obtained from the following...Ch. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Draw the products of the following reactions,...Ch. 13 - a. What five-carbon alkene forms the same product...Ch. 13 - Prob. 44PCh. 13 - Prob. 45PCh. 13 - Prob. 46PCh. 13 - Explain why the rate of bromination of methane...Ch. 13 - Prob. 48P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Define the situation when Is the Mechanism SN1 or SN2?arrow_forwardDoes increasing the energy barrier for an SN2 reaction increase or decrease the magnitude of the rate constant for the reactionarrow_forwardFill in Q,R,S,T,U and V with appropriate chemical reagants to make these reactions possible.arrow_forward
- Give the reaction mechanism for the following subparts in clear handwritten!arrow_forwardThe reaction of 3,4-dimethyl-3-hexanol (3,4-dimethylhexan-3-ol) (3R,4S configuration) with HBr (room temperature and concentrated) generates a mix of two stereoisomers. 1)Give the structure of the 2 compounds obtained in a wedge-wheel representation, specifying the absolute configuration. 2)Justify the formation of these 2 compounds using the mechanisms. 3) Give the expression for the reaction speed. 4) Is the final mixture obtained optically active? Justify your answer.arrow_forwardWhat would the orientation factor be for this reaction? Why? (>1, <1, or =1)arrow_forward
- Define Characteristics of the E2 Mechanism ?arrow_forwardHow can I solve this problem without the value of rate constant,k ? Any help is appreciated , thank you !arrow_forwardWhich step in this reaction mechanism for the iodination of acetone is the rate determining step and what are the rate laws for each of these elementary steps?arrow_forward
- What does the mechanism look like for 1,3-butadiene reacted with one mole of HBr to get 1-bromo-2-butene as a product?arrow_forward2,2-dimethylpropane only reacts with bromine in a solvent of CCl4. The presence of sunlight is required in this reaction. Specify the type of reaction and show the complete mechanism for the reaction occurred. This is a free radical halogenation, however I don't understand what it meant by "only recats with bromine in a solvent of CCL4"... can I have some explanation :(arrow_forwardBased on the information for the shown reactions, please indicate that the following statement is true or false: “Under standard conditions, the factor by which E1 increases the velocity of the reaction 1 is most likely to be lower than that by which E2 increases the velocity of the reaction 2”. Briefly explain your reasoning.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Kinetics: Initial Rates and Integrated Rate Laws; Author: Professor Dave Explains;https://www.youtube.com/watch?v=wYqQCojggyM;License: Standard YouTube License, CC-BY