
(a)
Interpretation:
A multistep synthesis of the given compounds from the given starting material has to be given.
Concept introduction:
Bromination:

2-methyl propane undergoes radical bromination which yields the 2-bromo-2-methylpropane.because bromination will occur where the tertiary radical is present. (bromination reactions are more selective reaction).
Formation of
The
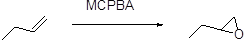
(b)
Interpretation:
A multistep synthesis of the given compounds from the given starting material has to be given.
Concept introduction:
Bromination:

2-methyl propane undergoes radical bromination which yields the 2-bromo-2-methylpropane.because bromination will occur where the tertiary radical is present. (bromination reactions are more selective reaction).
Oxidation of alcohol:
Alcohols reacts with hypochlorous (oxidizing agent) in the presence of acetic acid which yields the corresponding
Primary alcohols gives aldehyde, secondary alcohols gives ketone.
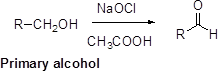
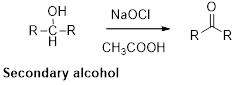
The alcohols reacts with acids like hydrochloric acid or hydrobromic acid, the bromine atom attacks back side of the carbon atoms which is bearing alcohol group and yields the corresponding product.
(c)
Interpretation:
A multistep synthesis of the given compounds from the given starting material has to be given.
Concept introduction:
Bromination:

2-methyl propane undergoes radical bromination which yields the 2-bromo-2-methylpropane.because bromination will occur where the tertiary radical is present. (bromination reactions are more selective reaction).
Oxidation of alcohol:
Alcohols reacts with hypochlorous (oxidizing agent) in the presence of acetic acid and yields the corresponding aldehyde and ketones.
Primary alcohols gives aldehyde, secondary alcohols gives ketone.
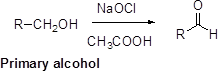
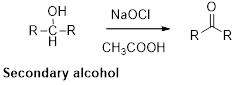
SN2 reaction:
The alcohols reacts with acids like hydrochloric acid or hydrobromic acid, the bromine atom attacks back side of the carbon atoms which is bearing alcohol group and yields the corresponding product.
(d)
Interpretation:
A multistep synthesis of the given compounds from the given starting material has to be given.
Concept introduction:
Bromination:

2-methyl propane undergoes radical bromination which yields the 2-bromo-2-methylpropane.because bromination will occur where the tertiary radical is present. (bromination reactions are more selective reaction).
Formation of epoxide:
The alkene can be converted to epoxide when alkene is treated with MCPBA (m-chloro perbenzoic acid)
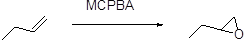
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Organic Chemistry
- Starting with benzene and other necessary reagents, design a synthesis for the following compound.arrow_forwarddraw an efficient synthesis strategy and show all products..arrow_forwardSynthesis. Use retrosynthetic analysis to describe how you would prepare the following compounds from the indicated reagents. You can use reagents that have no more than six carbons.arrow_forward
- For each of the following target molecules, design a multistep synthesis to show how it can be prepared from the given starting material:arrow_forwardThe synthesis shown above can be accomplished in three steps. Select the reagents for each step from the dropdown lists below.arrow_forwardCreate your own synthesis using minimum 6 of the following reagents.arrow_forward
- Show the step by step synthesis of the following compound.arrow_forwardShow a multistep synthesis to produce the product from the starting material shown.arrow_forwardComplete the following synthesis by selecting from the list of 10 reagents below. Each reagent (or set of reagents) is labeled as a letter. In the answer box, simply place the order of reagents used as uppercase letters. For example, if your synthesis involves using reagent A followed by B,followed by C, and then D, your answer would be: ABCD.arrow_forward
- Map out a multi-step synthesis for the transformation below, indicating specific reagents/products for each step.arrow_forwardPropose a multistep synthesis of the following target. All carbon atoms in the target must come from two molecules of propane. You can use any other reagents as needed. You do not have to draw any curved-arrow mechanism but are strongly encouraged to provide the products of each synthetic step.arrow_forwardI have proposed a suitable synthesis for the following transformation please let me know whether or not this is correct. If incorrect please let me know what I did wrong.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
