
Organic Chemistry
7th Edition
ISBN: 9780321803078
Author: Bruice, Paula Yurkanis
Publisher: Pearson College Div
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 37P
a. Propose a mechanism for the following reaction:
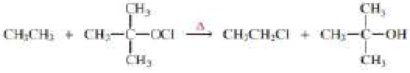
b. Given that ∆H° for the reaction is –42 kcal/mol and the bond dissociation enthalpies for the 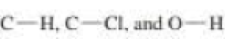 bonds are 101, 85, and 105 kcal/mol respectively, calculate the bond dissociation enthalpy of the
bonds are 101, 85, and 105 kcal/mol respectively, calculate the bond dissociation enthalpy of the 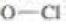 bond.
bond.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Resveratrol is an antioxidant found in the skin of red grapes. Its anticancer, anti-inflammatory, and various cardiovascular effects are under active investigation. (a) Draw all resonance structures for the radical that results from homolysis of the OH bond shown in red. (b) Explain why homolysis of this OH bond is preferred to homolysis of either OH bond in the other benzene ring.
give the reagents for parts a-p
What is a possible formula for a carbocation with m/z=97
Chapter 13 Solutions
Organic Chemistry
Ch. 13.2 - Prob. 1PCh. 13.2 - Write the steps for formation of...Ch. 13.3 - Prob. 3PCh. 13.4 - Prob. 4PCh. 13.5 - Prob. 7PCh. 13.5 - a. Would chlorination or bromination produce a...Ch. 13.5 - Prob. 10PCh. 13.6 - Prob. 11PCh. 13.7 - Prob. 12PCh. 13.7 - Prob. 13P
Ch. 13.8 - Prob. 14PCh. 13.8 - Draw the stereoisomers of the major...Ch. 13.9 - a. How many stereoisomers are formed from the...Ch. 13.9 - Prob. 17PCh. 13.9 - Prob. 19PCh. 13.9 - Prob. 20PCh. 13.9 - Prob. 21PCh. 13.10 - Prob. 22PCh. 13.11 - How many atoms share the unpaired electrons in...Ch. 13.11 - Prob. 24PCh. 13 - Prob. 25PCh. 13 - Prob. 26PCh. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 29PCh. 13 - Prob. 30PCh. 13 - Prob. 31PCh. 13 - Prob. 32PCh. 13 - Prob. 33PCh. 13 - Prob. 34PCh. 13 - Prob. 35PCh. 13 - Starting with cyclohexane, how could the following...Ch. 13 - a. Propose a mechanism for the following reaction:...Ch. 13 - What stereoisomers are obtained from the following...Ch. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Draw the products of the following reactions,...Ch. 13 - a. What five-carbon alkene forms the same product...Ch. 13 - Prob. 44PCh. 13 - Prob. 45PCh. 13 - Prob. 46PCh. 13 - Explain why the rate of bromination of methane...Ch. 13 - Prob. 48P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How do you identify if a reaction is Sn1 Sn2 E1 or E2? Draw a flowchart to identify if reactions are SN1 SN2 E1 or E2.arrow_forwardResveratrol is an antioxidant found in the skin of red grapes. Its anticancer, anti-inammatory, and various cardiovascular effects are under active investigation. (a) Draw all resonance structures for the radical that results from homolysis of the OH bond shown in red. (b) Explain why homolysis of this OH bond is preferred to homolysis of either OH bond in the other benzene ring.arrow_forwardTunicates are marine animals that are called "sea squirts" because when they are taken out of water, they tend to contract and expel seawater. Lepadiformine is a cytotoxic agent (toxic to cells) isolated from a marine tunicate. During a recent synthesis of lepadiformine, the investigators observed the formation of an interesting by-product (3) while treating diol 1 with a reagent similar in function to PBr3 (J. Org. Chem. 2012, 77, 3390–3400):arrow_forward
- These reagents can produce ketones with alkynes A. BH3, THF, H2O2 B. KMnO4 C. O3 D. H2SO4, H2O, HgSO4 choices:A,DB,CA,B,CA,B,C,Darrow_forwardChoose the best reagents from the list provided below for carrying out the following conversion. Match the reagent with the step number. HCl (aq), Zn(Hg) Br2, FeBr3 Na/NH3, -33 degrees C NBS, light KMnO4, H3O+ Mg metal, ether KOH, EtOH, heatarrow_forwardNicotinic acid, more commonly named niacin, is one of the B vitamins. Show how nicotinic acid can be converted to (a) ethyl nicotinate and then to (b) nicotinamide.arrow_forward
- With reference to the indicated C–H bonds in the following compound: a.Rank the C–H bonds in order of increasing bond strength. b.Draw the radical resulting from cleavage of each C–H bond, and classify it as 1°, 2°, or 3°. c. Rank the radicals in order of increasing stability. d.Rank the C–H bonds in order of increasing ease of H abstraction in a radical halogenation reaction.arrow_forwarda. Propose a mechanism for the following reaction: b. Given that ΔH° for the reaction is -42 kcal/mol and the bond dissociation enthalpies for the C¬H, C¬Cl, and O¬H bonds are 101, 85, and 105 kcal/mol respectively, calculate the bond dissociation enthalpy of the O¬Cl bond.arrow_forward1.Label kinetic product 2.Label the thermodynamic product 3.Predict the four products from reaction of alkene with one equivalent HBrarrow_forward
- The above reaction involves heterolytic bond breakage of HBr a) Encircle the nucleophile (s) and electrophile (s) and explain why. b) Give the mechanism of the reaction by: ) Drawing the appropriate arrows to track the flow of electrons in Showing the species formed after bond breakage and bond formation with appropriate charges. c) Predict the final products.arrow_forwardConsider 3-iodo-2,3-dimethylpentane and 3-iodo-2methylpentane. a. which reacts faster in an Sn2 reaction? Explain. b. which reacts faster in an E2 reaction? Explain.arrow_forward5..8. What is the expected major for the following reaction ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY