
Concept explainers
(a)
Interpretation:
The oxidized product of the following alcohol when oxidized with
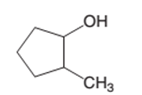
Concept Introduction:
A
In a chemical reaction, the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
The oxidation reaction is the reaction that involves the addition of O atom in the presence of certain oxidizing agents such as
Answer to Problem 64P
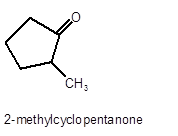
Explanation of Solution
To get the oxidized product of any alcohol, three steps must be followed:
- Locate the C atom in the parent chain that is bonded with −OH group.
- Convert that C atom to carbonyl C atom or
carboxylic acid as it is overall removal of H atoms. - Primary alcohols are oxidized to
aldehyde which further oxidized to a carboxylic acid. - Secondary alcohols are oxidized to
ketone (R2CO). - Tertiary alcohols are not oxidized as they do not have H atom on the C with the −OH group.
Hence, the oxidized of 2-methylcyclopnetanol will form 2-methylcyclopentanone molecule as 2-methylcyclopnetanol is a secondary alcohol.
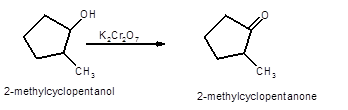
(b)
Interpretation:
The oxidized product of the following alcohol when oxidized with
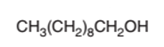
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction, the substance which is involved in conversion is said to be reactant, whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
The oxidation reaction is the reaction that involves the addition of O atom in the presence of certain oxidizing agents such as
Answer to Problem 64P
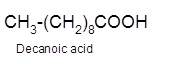
Explanation of Solution
To get the oxidized product of any alcohol, three steps must be followed:
- Locate the C atom in the parent chain that is bonded with −OH group.
- Convert that C atom to carbonyl C atom or carboxylic acid as it is overall removal of H atoms.
- Primary alcohols are oxidized to aldehyde which further oxidized to the carboxylic acid.
- Secondary alcohols are oxidized to ketone (R2CO).
- Tertiary alcohols are not oxidized as they do not have H atom on the C with the −OH group.
Hence, the oxidized of 1-decanol will form decanoic acid molecule as 1-decanol is a primary alcohol.
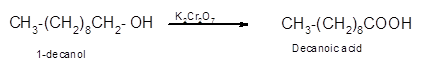
(c)
Interpretation:
The oxidized product of following alcohol when oxidized with
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction; the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
The oxidation reaction is the reaction that involves the addition of O atom in the presence of certain oxidizing agents such as
Answer to Problem 64P

Explanation of Solution
To get the oxidized product of any alcohol, three steps must be followed:
- Locate the C atom in the parent chain that is bonded with −OH group.
- Convert that C atom to carbonyl C atom or carboxylic acid as it is overall removal of H atoms.
- Primary alcohols are oxidized to aldehyde which further oxidized to the carboxylic acid.
- Secondary alcohols are oxidized to a ketone (R2CO).
- Tertiary alcohols are not oxidized as they do not have H atom on the C with the −OH group.
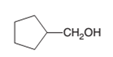
Hence, the oxidized of cyclopentylmethanol will form cyclopentanecarboxylic acid molecule as cyclopentylmethanol is a primary alcohol.
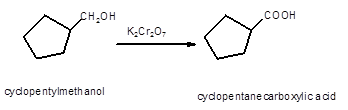
(d)
Interpretation:
The oxidized product of following alcohol when oxidized with
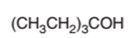
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction; the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
Oxidation reaction is the reaction that involves the addition of O atom in the presence of certain oxidizing agents such as
Answer to Problem 64P
2-ethyl-3-pentanol cannot oxidize as it is a tertiary alcohol.
Explanation of Solution
To get the oxidized product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Convert that C atom to carbonyl C atom or carboxylic acid as it is the overall removal of H atoms.
- Primary alcohols are oxidized to aldehyde which further oxidized to the carboxylic acid.
- Secondary alcohol is oxidized to a ketone (R2CO).
- Tertiary alcohols are not oxidized as they do not have H atom on the C with the −OH group.
Hence 2-ethyl-3-pentanol cannot oxidize as it is a tertiary alcohol.
Want to see more full solutions like this?
Chapter 14 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Draw the products formed when each alcohol is oxidized with K 2Cr 2O 7. In some cases, no reaction occurs.arrow_forward1. What functional group is produced when an aldehyde reacts with H2/Pt? A.secondary alcohol B. carboxylic acid C.hemiacetal D. primary alcohol E.alkane F.tertiary alcohol G. alkene 2. What reaction occurs when an aldehyde reacts with H2/Pt to form a primary alcohol? A. Hydration B. Hydration C. Dehydration D. Oxidation E. Reduction( hydrogentation) 3. What reaction occurs when an Ester react with H+/H2O to from a carboxylic acid and alcohol? A. Dehydration B. Reduction ( Hydrogenation) C.Hydrolysis D. Hydration E.oxidationarrow_forwardDraw the product formed when the alcohol cyclobutanol is dehydrated with H2SO4.arrow_forward
- 1) Draw each alcohol2) Categorize the alcohol as primary, secondary or tertiary3) Oxidize each alcohol as many times as possible4) Name the product(s) if there are any molecule: 3-Tertbutylcyclopropanolarrow_forward1. O-hydroxybenzoic acid is a major product formed with phenol and which other reactant/s I-primary alcohol II-sodium hydroxide III-water IV-carbon dioxide A.I and III B. I and IV C. II and III D. II and IVarrow_forwardIsopropyl alcohol is Select one: a. CH3CH2OH b. CH3CH(OH)CH3 c. CH3CH2CH2OH d. CH3OHarrow_forward
- ALCOHOLS 1. WHY IS ETHANOL MORE SOLUBLE IN WATER THAN 1-HEXANOL? 2. WHAT IS DENATURED ALCOHOL? AND WHY IS ALCOHOL DENATURED? ETHER 1. WHY DOES DIETHYL ETHER HAVE MUCH LOWER BOILING POINT THAN 1-BUTANOL?arrow_forwardWhich one is more soluble in octane, C8H18: Propane CH3-CH2-CH3 or 1-Propanol, CH3-CH2-CH2-OH. Why ? A. 1-propanol because it has strong dispersion forces. B. 1-propanol because it is polar C. 1-propanol because it can hydrogen bond D. Propane because it has only dispersion forcesarrow_forwardWhen heated with H2SO4, both 3,3-dimethyl-2-butanol and 2,3-dimethyl-2-butanol are dehydrated to form 2,3-dimethyl-2-butene. Which alcohol dehydrates more rapidly?arrow_forward
- Give a systematic (IUPAC) name for each diol.(a) CH3CH(OH)(CH2)4CH(OH)C(CH3)3arrow_forward3. Dimethyl ether, H3C-O-CH3, is a gas at room temperature and insoluble in water. Draw a structural isomer of dimethyl ether that is much more soluble in water and explain the basis of its increased water solubility.arrow_forwardShow how each alcohol or diol can be prepared from an alkene. (a) 2-Pentanol (b) 1-Pentanol (c) 2-Methyl-2-pentanol (d) 2-Methyl-2-butanol (e) 3-Pentanol (f) 3-Ethyl-3-pentanol (g) 1,2-Hexanediolarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





