
(a)
Interpretation:
The following
CH3CH2CH2CH2CH2Br
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds are said to be
Primary alkyl halide has one carbon bonded to the carbon atom that is bonded with halogen atom. Secondary alkyl halide has two carbons bonded to the same carbon atom bonded with the halogen. A tertiary alkyl halide has three carbons bonded to the same carbon atom bonded with the halogen atom.
(b)
Interpretation:
The following alkyl halide should be classified as primary, secondary and tertiary:
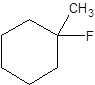
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds are said to be organic chemistry. The molecular formula of an organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule.
Primary alkyl halide has one carbon bonded to the carbon atom that is bonded with a halogen atom. Secondary alkyl halide has two carbons bonded to the same carbon atom bonded with the halogen. A tertiary alkyl halide has three carbons bonded to the same carbon atom bonded with the halogen atom.
(c)
Interpretation:
The following alkyl halide should be classified as primary, secondary and tertiary:
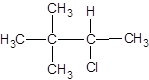
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds are said to be organic chemistry. The molecular formula of an organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule.
Primary alkyl halide has one carbon bonded to the carbon atom that is bonded with the halogen atom. Secondary alkyl halide has two carbons bonded to the same carbon atom bonded with the halogen. A tertiary alkyl halide has three carbons bonded to the same carbon atom bonded with the halogen atom.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Give a systematic (IUPAC) name for each diol. ) HO¬(CH2)8¬OHarrow_forward1. Which alcohol has a higher boiling point?a. (i) 2-methylpropan-2-ol or (ii) butan-2-olb. (i) hexan-1-ol or (ii) 3,3-dimethylbutan-1-olarrow_forwardWhat alkenes are formed when each alcohol is treated with H 2SO 4? Use the Zaitsev rule to predict the major product.arrow_forward
- 1. O-hydroxybenzoic acid is a major product formed with phenol and which other reactant/s I-primary alcohol II-sodium hydroxide III-water IV-carbon dioxide A.I and III B. I and IV C. II and III D. II and IVarrow_forward8. What is the IUPAC name for the molecule shown below? a 1,2-dimethyl-1-propylcyclopropane b 2,4-dimethyl-1-propylcyclopropane c 1,2-dimethyl-3-propylcyclopropane d 2,4-dimethyl-3-propylcyclopropanearrow_forwardDraw the products formed when each alcohol is dehydrated with H 2SO 4. Use the Zaitsev rule to predict the major product when a mixture forms.arrow_forward
- What alkenes are formed when each alcohol is dehydrated with TsOH? Label the major product when a mixture resultsarrow_forward___ is an example of an alkyl halide. Select one: a. KCl b. CHCl3 c. NaCl d. CF2=CF2arrow_forwardGive the structure corresponding to each IUPAC name. 2,4 dimethyl- 2 hexanolarrow_forward
- Identify the IUPAC name of the given structure. A. 2 - methylhexan-5-one B. 5 - methylhexan-2-one C. 2 - heptanone D. 5 - heptanone Identify the IUPAC name of the given structure. A. 4 - bromopentan-3-one B. 1 - bromobutan-2-one C. 2 - bromobutan-one D. None of the abovearrow_forwardWhat reagent is necessary to complete the reaction? CH3-CH2-CH-C-OH ? CHỊCH,CH C-0- Na* CH3 CH3 NaO O NaCl O Na O NaOH + H₂Oarrow_forwardPhenol + Br2/H2Oarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
