
a)
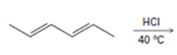
Interpretation:
The major products formed during the addition of one equivalent of HCl to hexa-2, 4-diene along with the mechanism of their formation is to be shown.
Concept introduction:
Conjugated dienes undergo electrophilic addition reactions through the formation of an allyl carbocation. The allyl cation is resonance stabilized and the attack of chloride ion on each of these forms leads to the formation of a mixture of 1, 2- and 1, 4-addition products.
To show:
The major product formed during the addition of one equivalent of HCl to hexa-2, 4-diene along with the mechanism of their formation.
b)
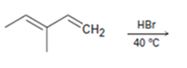
Interpretation:
The major products formed during the addition of one equivalent of HX to 3-methylpenta-1, 3-diene along with the mechanism of their formation is to be shown.
Concept introduction:
Conjugated dienes undergo electrophilic addition reactions through the formation of an allyl carbocation. The allyl cation is resonance stabilized and the attack of chloride ion on each of these forms leads to the formation of a mixture of 1, 2- and 1, 4-addition products.
To show:
The major products formed during the addition of one equivalent of HBr to 3-methylpenta-1, 3-diene along with the mechanism of their formation.
c)
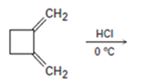
Interpretation:
The major product formed during the addition of one equivalent of HCl to 1, 2-dimethylenecyclobutane, along with the mechanism of their formation is to be shown.
Concept introduction:
Conjugated dienes undergo electrophilic addition reactions through the formation of an allyl carbocation. The allyl cation is resonance stabilized and the attack of chloride ion on each of these forms leads to the formation of a mixture of 1, 2- and 1, 4-addition products.
To show:
The major product formed during the addition of one equivalent of HCl to 1, 2-dimethylenecyclobutane along with the mechanism of their formation.
Trending nowThis is a popular solution!

Chapter 14 Solutions
Organic Chemistry
- Provide the mechanism and products for the acid-catalyzed epoxide opening reactions below, including appropriate stereochemistry.arrow_forwardIn light of the fact that tertiary alkyl halides undergo spontaneous dissociation to yield a carbocation plus halide ion (see Problem 10-45), propose a mechanism for the following reaction.arrow_forwardPredict the major product and show the complete mechanism for each electrophilicreaction below.arrow_forward
- What is the major product for the following reaction? Show the mechanism of the reaction.arrow_forwardDraw the products and show the mechanism for the reactions below. Between each reactionpair indicate which one will proceed faster and explain whyarrow_forwardwhat is the expected major product of HBr addition to the alkene shown below? Show the mechanismarrow_forward
- Please give a detailed stepwise mechanism for the following reactions of Q.1 and Q.2. All arrows, charges, intermediates, and resonance structures must be shown.arrow_forwardShow the mechanism of the following reaction to obtain the product of 2 moles of an alkyl halide: 2 moles of H-Br + R-C-O-C-R = R-C-X + R-C-X + H2Oarrow_forward
