
Concept explainers
a)
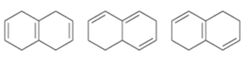
Interpretation:
The molecules given are to be arranged from shortest to longest wavelength on the basis their wavelength of maximum absorption in UV spectroscopy.
Concept introduction:
Extended conjugation shifts the UV absorption maxima to higher wavelengths. For example, the UV absorption maxima for 1,3-butadiene is 217 nm and that of 1,3,5-hexatriene is 258nm.
To arrange:
The molecules given from shortest to longest wavelength on the basis their wavelength of maximum absorption in UV spectroscopy.
b)
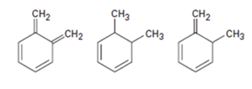
Interpretation:
The molecules given are to be arranged from shortest to longest wavelength on the basis their wavelength of maximum absorption in UV spectroscopy.
Concept introduction:
Extended conjugation shifts the UV absorption maxima to higher wavelengths. For example, the UV absorption maxima for 1,3-butadiene is 217 nm and that of 1,3,5-hexatriene is 258nm.
To arrange:
The molecules given from shortest to longest wavelength on the basis their wavelength of maximum absorption in UV spectroscopy.
c)
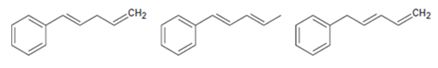
Interpretation:
The molecules given are to be arranged from shortest to longest wavelength on the basis their wavelength of maximum absorption in UV spectroscopy.
Concept introduction:
Extended conjugation shifts the UV absorption maxima to higher wavelengths. For example, the UV absorption maxima for 1,3-butadiene is 217 nm and that of 1,3,5-hexatriene is 258nm.
To arrange:
The molecules given from shortest to longest wavelength on the basis their wavelength of maximum absorption in UV spectroscopy.
Trending nowThis is a popular solution!

Chapter 14 Solutions
Organic Chemistry
- Which of the following is most UNLIKELY to absorb in the UV-Vis region? a. chlorophyll b. heptane c. nitrophenol d. beta-carotenearrow_forwardPlease help me! Part 3A Set 1. Can IR spectroscopy be used to differentiate between the two compounds? Briefly explain why or why not. What significant absorptions would be observed in the IR spectrum?arrow_forwardHow would infrared spectroscopy be useful in distinguishing between the followingcompounds? Cite absorption bands you would expect from each.compounds?arrow_forward
- Which compound from the list below can undergo a Diels-Alder reaction?arrow_forwardCompounds B and C are isomers with molecular formula C5H9BrO2. The 1H NMR spectrum of compounds B and C are shown below. The IR spectrum corresponding to compound B showed strong absorption bands at 1739, 1225, and 1158 cm-1, while the spectrum corresponding to compound C have strong bands at 1735, 1237, and 1182 cm-1. 1.Based on the information provided, determine the structure of compounds B and C. 2.Assign all peaks in 1H NMR spectrum of compounds B and C.arrow_forwardInfrared Interpretation – interpret all absorptions in the 4000-1400 cm-1 region of the IR spectra of 2-methyl-4-heptanol. Label the recorded IR spectra and provide an indication of the impurities present, if any.arrow_forward
- Part 3A Set 1. Can IR spectroscopy be used to differentiate between the two compounds? Briefly explain why or why not. What significant absorptions would be observed in the IR spectrum?arrow_forwardWHich of these molecules best corresponds to IR spectrum shown. and whyarrow_forwardPropose a structure consistent with each set of data.a.) C9H10O2: IR absorption at 1718 cm−1 b.) C9H12: IR absorption at 2850–3150 cm−1arrow_forward
- Part IIDraw the structure of the synthesized product (do not include side or by products) from experiments performed in Chem 272L. How can IR spectroscopy be used to monitor the progress of each of the following reactions (if reaction went to completion and/or check the purity of the final product)? What absorptions would be observed in the IR spectrum? And if IR spectroscopy could not have been used, briefly explain why.arrow_forwardWich of these molecules best corresponds to IR spectrum shown. and whyarrow_forwardcompound with formula C9H10O. If the unknown has an IR absorption at 1690 cm–1 please tell me structure ??arrow_forward
