
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14.SE, Problem 17VC
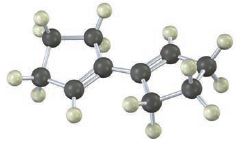
Show the product of the Diels–Alder reaction of the following diene with 3-buten-2-one, H2C = CHCOCH3. Make sure you show the full stereochemistry of the reaction product.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
a. How many linear dienes have molecular formula C6H10? (Disregard cis–trans isomers.) b. How many of the linear dienes in part a are conjugated dienes? c. How many are isolated dienes? d. How many are cumulated dienes?
Alkenes can be hydrated to form alcohols by (1) hydroboration followed by oxidation with alkaline hydrogen peroxide and (2) acid-catalyzed hydration. Compare the product formed from each alkene by sequence (1) with those formed from (2).
Q.)Cyclopentene
Predict the organic products of the reaction of 2 butene with each reagent. Be sure to indicate stereochemistry and regioselectivity where appropriate. a. Br2 in H2O
b. Hg(OAc)2, H2O
c. Product from (b) + NaBH4
Chapter 14 Solutions
Organic Chemistry
Ch. 14.1 - Prob. 1PCh. 14.2 - Give the structures of both 1, 2 and 1, 4 adducts...Ch. 14.2 - Prob. 3PCh. 14.2 - Give the structures of both 1, 2 and 1, 4 adducts...Ch. 14.3 - Prob. 5PCh. 14.3 - Prob. 6PCh. 14.5 - Predict the product of the following Diels–Alder...Ch. 14.5 - Prob. 8PCh. 14.5 - Which of the following dienes have an s-cis...Ch. 14.5 - Predict the product of the following Diels–Alder...
Ch. 14.6 - Prob. 11PCh. 14.6 - Prob. 12PCh. 14.7 - Prob. 13PCh. 14.7 - Prob. 14PCh. 14.8 - Which of the following compounds would you expect...Ch. 14.SE - Prob. 16VCCh. 14.SE - Show the product of the Diels–Alder reaction of...Ch. 14.SE - Prob. 18VCCh. 14.SE - Prob. 19VCCh. 14.SE - Prob. 20MPCh. 14.SE - Prob. 21MPCh. 14.SE - In light of your answer to Problem 14-21 propose...Ch. 14.SE - Luminol, which is used by forensic scientists to...Ch. 14.SE - Prob. 24MPCh. 14.SE - Give IUPAC names for the following compounds:Ch. 14.SE - Prob. 26APCh. 14.SE - Prob. 27APCh. 14.SE - Electrophilic addition of Br2 to isoprene...Ch. 14.SE - Prob. 29APCh. 14.SE - Prob. 30APCh. 14.SE - Predict the products of the following...Ch. 14.SE - 2,3-Di-tert-butyl-1,3-butadiene does not undergo...Ch. 14.SE - Prob. 33APCh. 14.SE - Prob. 34APCh. 14.SE - Prob. 35APCh. 14.SE - Prob. 36APCh. 14.SE - Rank the following dienophiles in order of their...Ch. 14.SE - Prob. 38APCh. 14.SE - Prob. 39APCh. 14.SE - Prob. 40APCh. 14.SE - Although the Diels–Alder reaction generally...Ch. 14.SE - Prob. 42APCh. 14.SE - Tires whose sidewalls are made of natural rubber...Ch. 14.SE - Prob. 44APCh. 14.SE - Prob. 45APCh. 14.SE - Prob. 46APCh. 14.SE - Would you expect allene, H2C = C = CH2, to show a...Ch. 14.SE - The following ultraviolet absorption maxima have...Ch. 14.SE - Prob. 49APCh. 14.SE - -Ocimene is a pleasant-smelling hydrocarbon found...Ch. 14.SE - Draw the resonance forms that result when the...Ch. 14.SE - Prob. 52APCh. 14.SE - Treatment of 3,4-dibromohexane with strong base...Ch. 14.SE - Prob. 54APCh. 14.SE - Prob. 55APCh. 14.SE - Prob. 56APCh. 14.SE - Prob. 57APCh. 14.SE - Prob. 58APCh. 14.SE - Hydrocarbon A, C10H14, has a UV absorption at...Ch. 14.SE - Prob. 60APCh. 14.SE - Prob. 61APCh. 14.SE - Prob. 62APCh. 14.SE - Prob. 63APCh. 14.SE - Prob. 64APCh. 14.SE - The double bond of an enamine (alkene + amine) is...Ch. 14.SE - Prob. 66AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Bicyclo-2,5-heptadiene can be prepared in two steps from cyclopentadiene and vinyl chloride. Provide a mechanism for each step.arrow_forwardShow the starting diene and dienophile you could use to prepare the following molecule:arrow_forwardReaction of HBr with 2-methylpropene yields 2-bromopropane. What is the structure of the carbocation intermediate formed during the reaction? Show the mechanism of the reaction.arrow_forward
- Show the product, including stereochemistry, of the reaction of the epoxide below with Na+ -CN, H2O.arrow_forwardReaction of HBr with 2-methylpropene yields 2-bromo-2-methylpropane. What is the structure of the carbocation formed during the reaction? Show the mechanism of the reaction.arrow_forwardWhat products would you expect from reaction of the following alkenes with NBS? If more than one product is formed, show the structures of all.arrow_forward
- Predict the major products of the following reactions.a.4@chlorocycloheptene + Hg(OAc)2 in CH3OH b.the product from part (c), treated with NaBH4arrow_forwardDraw the products formed when the following diene is treated with O3 followed by CH3SCH3.arrow_forwardIn an electrophilic addition reaction, conjugated dienes give more than one expected product. Show the products when using the reagent 1,3 –butadiene and HBr.arrow_forward
- If this diene were to be treated with HBr at -10C, where would the Br attach to the structure in the major product?arrow_forwardReaction of hbr with 2-methylpropene yields 2 bromo 2 methylpropane. What is the structure of the carbocation formed during the reaction? Show the mechanism of reaction.arrow_forwardWhat product will you get when you treat trans-2-butene with HCl/H2Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY