
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 17, Problem 17.11P
Interpretation Introduction
Interpretation: The structural formula for monopotassium oxalate has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
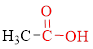
Carboxylic acid salts are the neutral compounds which are formed by the reaction of carboxylic acid with a strong base.
The Structural formula of a compound shows how the atoms are arranged in a molecule and, in particular, shows which functional groups are present.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Oxalic acid is found in the leaves of rhubarb, primarilyin the form of the calcium salt. Since high levels of oxalicacid are toxic, only rhubarb stalks are used to make strawberry rhubarb pie. What is the IUPAC name of oxalic acid?Write the structure of the calcium salt of oxalic acid.
What reagent serves as the source of ammonia to deprotonate oxalic acid in the production of calcium oxalate monohydrate?
a. ammonium oxalate
b. hydrochloric acid
c. urea
d. ammonium hydroxide
When methanitrobenzoic acid is reacted with bromomethane in the presence of Lewis acids, which product occur.
Chapter 17 Solutions
Organic Chemistry
Ch. 17.2 - Prob. 17.1PCh. 17.4 - Which is the stronger acid in each pair?Ch. 17.4 - Prob. 17.3PCh. 17.7 - Prob. 17.4PCh. 17.8 - Prob. 17.5PCh. 17.8 - Prob. AQCh. 17.8 - Prob. BQCh. 17.8 - Prob. CQCh. 17.8 - Permethrin and Bifenthrin Pyrethrin is a natural...Ch. 17.9 - Prob. 17.6P
Ch. 17 - Write the IUPAC name of each compound, showing...Ch. 17 - Prob. 17.8PCh. 17 - Prob. 17.9PCh. 17 - Prob. 17.10PCh. 17 - Prob. 17.11PCh. 17 - Prob. 17.12PCh. 17 - Prob. 17.13PCh. 17 - On a cyclohexane ring, an axial carboxyl group has...Ch. 17 - Prob. 17.15PCh. 17 - Prob. 17.16PCh. 17 - Prob. 17.17PCh. 17 - Complete each reaction.Ch. 17 - Prob. 17.19PCh. 17 - Prob. 17.20PCh. 17 - Prob. 17.21PCh. 17 - Show the reagents and experimental conditions...Ch. 17 - Prob. 17.23PCh. 17 - Prob. 17.24PCh. 17 - Prob. 17.25PCh. 17 - In each set, assign the acid its appropriate pKa.Ch. 17 - Low-molecular-weight dicarboxylic acids normally...Ch. 17 - Complete the following acid-base reactions. (a)...Ch. 17 - Prob. 17.29PCh. 17 - Prob. 17.30PCh. 17 - Excess ascorbic acid is excreted in the urine, the...Ch. 17 - Give the expected organic product when...Ch. 17 - Show how to convert trans-3-phenyl-2-propenoic...Ch. 17 - Show how to convert 3-oxobutanoic acid...Ch. 17 - Prob. 17.35PCh. 17 - Prob. 17.36PCh. 17 - Prob. 17.37PCh. 17 - When 4-hydroxybutanoic acid is treated with an...Ch. 17 - Fischer esterification cannot be used to prepare...Ch. 17 - Draw the product formed on thermal decarboxylation...Ch. 17 - Prob. 17.41PCh. 17 - Show how cyclohexanecarboxylic acid could be...Ch. 17 - Prob. 17.43PCh. 17 - Prob. 17.44PCh. 17 - Prob. 17.45PCh. 17 - Write the products of the following sequences of...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Prob. 17.51PCh. 17 - Complete the following Fischer esterification...Ch. 17 - Prob. 17.53P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. Illustrate with equation: Laboratory preparation of acetic acid Laboratory preparation of benzoic acid Laboratory preparation of salicylic acid Reduction of monocarboxylic acid Oxidation of monocarboxylic acid 2. Give three uses each: Acetic acid Benzoic acid Salicylic acidarrow_forwardDraw the structure of m-chlorobenzoic acid :arrow_forwardIf you attempted to make soap from butanoic acid by forming sodium butanoate, would your preparation have the properties to act as an emulsifying agent (soap)?arrow_forward
- Sulphuric acid catalyzes the reaction between compound D and compound E producing propl hexanoate. This reaction is an example of Fischer esterification. Compound D reacts with phosphorus tribromide to give alkyl bromide. Draw the structure and give the name of compound D and Earrow_forwardWhat reagents would you use to chemically extract benzoic acid and valeric acid? Or can it not be chemically extracted? The image shows the compound structure.arrow_forwardBisphenol A is widely used as a building block in polymer synthesis and is found in the polycarbonate hard plastics of reusable drink containers, DVDs, cell phones, and other consumer goods. Bisphenol A is reported to have estrogenic activity, and its widespread occurrence in our environment is a potential concern. Describe one or two biochemical experiments that could be done to compare the activity of bisphenol A with that of its estradiol, its structural relative.arrow_forward
- 1. Arrange the test samples in order of increasing acidity? Explain briefly. -Ethanoic acid, Butanoic acid, Benzoic acid, Salicylic acid 2. Arrange the test samples in order of increasing reactivity with sodium bicarbonate? Explain briefly -Ethanoic acid, Butanoic acid, Benzoic acid 3. Arrange the test samples in order of increasing solubility in water? Explain briefly. -Ethylamine, Diethylamine, Aniline 4. Arrange the test samples in order of increasing basicity? Explain briefly. -NaOH, Ethylamine, Anilinearrow_forwardWhy is dimethylamine more basic than aniline? (Provide structures)arrow_forwardDigitalis is a preparation made from the dried seeds and leaves of the purple foxglove, Digitalis purpurea, a plant native to southern and central Europe and cultivated in the United States. The preparation is a mixture of several active components, including digitalin. Digitalis is used in medicine to increase the force of myocardial contraction and as a conduction depressant to decrease heart rate (the heart pumps more forcefully but less often).arrow_forward
- Distinguish between Acid anhydride and Basic anhydridearrow_forwardFeatures of the structure of imino acids. Formation of amides from amino acids.arrow_forwardProvide the chemical name and common name for Follicle Stimulating Hormone Describe the molecular formula and structural formula for Follicle Stimulating Hormonearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
