
Concept explainers
What ester is formed when butanoic acid
a.
b.
c.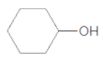
d.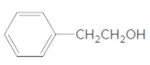
(a)
Interpretation:
The ester formed on treating butanoic acid with CH3OH in the presence of H2SO4 should be determined.
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 55P
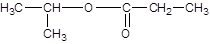
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between CH3CH2CH2COOH (butanoic acid) and methanol is:
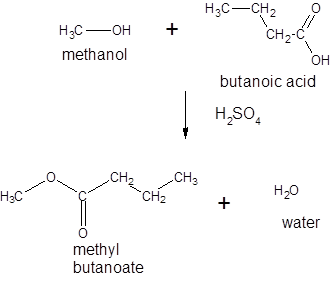
(b)
Interpretation:
The ester formed on treating butanoic acid with CH3CH2CH2OH in the presence of H2SO4 should be determined.
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 55P
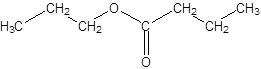
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between CH3CH2CH2COOH (butanoic acid) and propanol is:
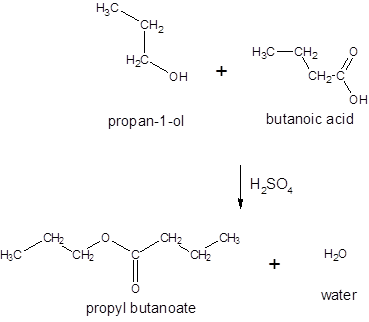
(c)
Interpretation:
The ester formed on treating butanoic acid with following alcohol in the presence of H2SO4 should be determined:
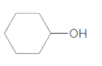
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 55P
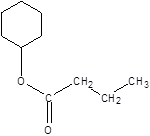
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between CH3CH2CH2COOH (butanoic acid) and cyclohexanol is:
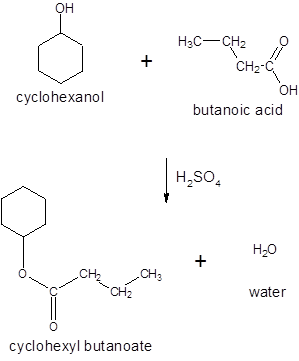
(d)
Interpretation:
The ester formed on treating butanoic acid with following alcohol in the presence of H2SO4 should be determined:
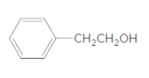
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 55P
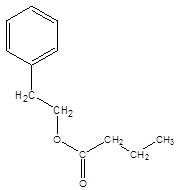
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between CH3CH2CH2COOH (butanoic acid) and 2-phenylethan-1-ol is:
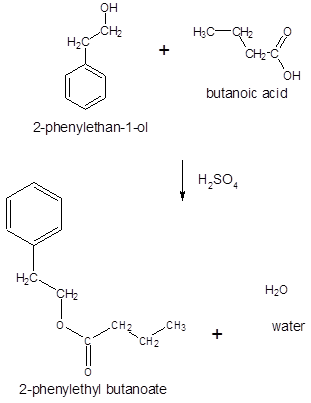
Want to see more full solutions like this?
Chapter 17 Solutions
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
- What carbonyl compound is needed to make each alcohol by a reduction reaction? What carbonyl compound is needed to make each alcohol by a reduction reaction?arrow_forwardMCQ 3: The carbonyl group in aldehyde is A. C=O B. C-O C. CO D. CHOarrow_forwardtaken in order to gor the product Please explain the mechanisms/stepsarrow_forward
- Give the IUPAC name for each ketone. C-CHCH,CH3 b. a.arrow_forwardDraw the products formed when each alcohol undergoes dehydration with TsOH, and label the major product when a mixture results.arrow_forwardWhat alkenes are formed when each alcohol is treated with H 2SO 4? Use the Zaitsev rule to predict the major product.arrow_forward
- What products are formed when each alcohol is oxidized with K2Cr2O7? a. CH3CH2CH2CH2CH2OHarrow_forwardDraw the products formed when each alcohol is dehydrated with H 2SO 4. Use the Zaitsev rule to predict the major product when a mixture forms.arrow_forwardWhich alcohols can be prepared as a single product by hydroboration– oxidation of an alkene? Which alcohols can be prepared as a single product by the acid-catalyzed addition of H2O to an alkene?arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning


