
Concept explainers
(a)
Interpretation:
The products formed when the following ester is treated with H2O and H2SO4 should be determined:
Concept Introduction:
Alcohols are the organic compounds with general chemical formula of R-OH whereas carboxylic acids are the organic molecules with R-COOH as general chemical formula.
(b)
Interpretation:
The products formed when the following ester is treated with H2O and H2SO4 should be determined:
Concept Introduction:
Functional groups are the groups of atoms or atoms which are bonded with parent carbon chain in the organic molecule and are responsible for the physical and chemical properties of the compound. In organic chemistry, there are different functional groups such as carboxylic acid, alcohol, ester, or amide.
Alcohols are the organic compounds with general chemical formula of R-OH whereas carboxylic acids are the organic molecules with R-COOH as general chemical formula.
(c)
Interpretation:
The products formed when the following ester is treated with H2O and H2SO4 should be determined:
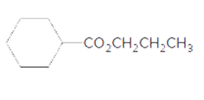
Concept Introduction:
Functional groups are the groups of atoms or atoms which are bonded with parent carbon chain in the organic molecule and are responsible for the physical and chemical properties of the compound. In organic chemistry, there are different functional groups such as carboxylic acid, alcohol, ester, or amide.
Alcohols are the organic compounds with general chemical formula of R-OH whereas carboxylic acids are the organic molecules with R-COOH as general chemical formula.
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
- Safrole is a naturally occurring acetal isolated from sassafras plants. Once used as a common food additive in root beer and other beverages, it is now banned because it is carcinogenic. What compounds are formed when safrole is hydrolyzed with aqueous acid? safrolearrow_forwardWhat products are formed when X, which contains both a lactone and an acetal, is treated with each reagent: (a) H,O*; (b) NaOH, H,O? Xarrow_forwardWhat ester and Grignard reagent are needed to prepare each alcohol?arrow_forward
- Which of the following are a. hemiacetals? b. acetals? c. hydrates?arrow_forwardCompound that is most easily hydrolyzed by acid in water `NH A. В. F C. D.arrow_forwardFenfluramine and phentermine are two components of fen–phen, an appetite suppressant withdrawn from the market in 1997 after it was shown to damage the heart valves in some patients. What products are formed when fenfluramine and phentermine are each treated with acetic acid (CH3CO2H)?arrow_forward
- Which is propyl propanoate? A. CH₂CH₂CH₂OOCCH₂CH; B. CH₂CH₂CH₂COOCH₂CH₂ C. CH₂CH₂CH₂COCH₂CH₂ D. CHỊCH,CH,OCH,CHỊCH, A B C Darrow_forwardDraw the products formed when phenol(C6H5OH) is treated with each reagent. Give an explanation. e. Br2, FeBr3 f. Br2arrow_forward1. Draw the product formed when phenylacetaldehyde (C6H5CH2CHO) is treated with each reagent:arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
