
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 19, Problem 19.43P
Interpretation Introduction
Interpretation:
N, N-diethyl m-toluamide (DEET) is the active ingredient in several common insect repellents. The acid and
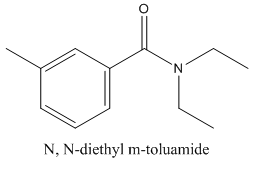
Concept Introduction:
Amides are formed by a reaction with a carboxylic acid and an amine which are the functional groups. This results in the formation of amide bond, in which OH group of carboxylic acid reacts with one of the H in amine group and CO-NH bond is formed. This bond is known as an amide bond.
The mechanism for formation of an amide is as follows taking example of propanoic acid and ethylamine:
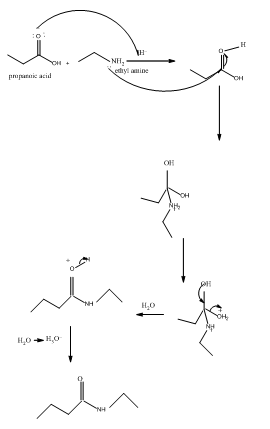
The hydrolysis of amide so formed can give back the carboxylic acid and amine.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What carboxylic acid and amine are needed to synthesize the pain reliever phenacetin? Phenacetin was once a component of the over-the-counter pain reliever APC (aspirin, phenacetin, caffeine), but it is no longer used because of its kidney toxicity.
Draw the products formed when each amine is treated with [1] CH3I (excess); [2] Ag2O; [3] Δ. Indicate the major product when a mixture results.
Arrange the compounds in order of decreasing basicity
A. ethylamine
B. 2-aminoethanol
C. 3-amino-1-propanol
Chapter 19 Solutions
Introduction to General, Organic and Biochemistry
Ch. 19.1 - Prob. 19.1PCh. 19.4 - Problem 19-2 Complete the equation for each...Ch. 19.4 - Prob. 19.3PCh. 19 - Prob. 19.4PCh. 19 - Write the IUPAC name for each compound.Ch. 19 - Prob. 19.6PCh. 19 - Prob. 19.7PCh. 19 - Prob. 19.8PCh. 19 - Prob. 19.9PCh. 19 - 0 Complete the equations for these reactions.
Ch. 19 - Prob. 19.11PCh. 19 - Prob. 19.12PCh. 19 - Prob. 19.13PCh. 19 - Prob. 19.14PCh. 19 - Prob. 19.15PCh. 19 - 6 Why are Dacron and Mylar referred to as...Ch. 19 - 7 What type of structural feature do the...Ch. 19 - Prob. 19.18PCh. 19 - Prob. 19.19PCh. 19 - 0 Show how triphosphoric acid can form from three...Ch. 19 - 1 Write an equation for the hydrolysis of...Ch. 19 - 2 (Chemical Connections 19A) Locate the ester...Ch. 19 - Prob. 19.23PCh. 19 - Prob. 19.24PCh. 19 - Prob. 19.25PCh. 19 - Prob. 19.26PCh. 19 - Prob. 19.27PCh. 19 - 8 (Chemical Connections 19C) Once it has been...Ch. 19 - Prob. 19.29PCh. 19 - Prob. 19.30PCh. 19 - Prob. 19.31PCh. 19 - Prob. 19.32PCh. 19 - Prob. 19.33PCh. 19 - 4 (Chemical Connections 19F) Why do Lactomer...Ch. 19 - Prob. 19.35PCh. 19 - Prob. 19.36PCh. 19 - Prob. 19.37PCh. 19 - 8 In Chapter 22, we will discuss a class of...Ch. 19 - Prob. 19.39PCh. 19 - Prob. 19.40PCh. 19 - Prob. 19.41PCh. 19 - Prob. 19.42PCh. 19 - Prob. 19.43PCh. 19 - Prob. 19.44PCh. 19 - Prob. 19.45PCh. 19 - Prob. 19.46PCh. 19 - Prob. 19.47PCh. 19 - Prob. 19.48PCh. 19 - Prob. 19.49P
Knowledge Booster
Similar questions
- Given that C6H11COOH has a pKa = 4.8 and C6H11N+H3 has a pKa = 10.7, (a) What pH would you make the water layer to cause the carboxylic acid to dissolve in the water layer and the amine to dissolve in the ether layer? (b) What pH would you make the water layer to cause the carboxylic acid to dissolve in the ether layer and the amine to dissolve in the water layer?arrow_forward3 Would the product be a(n) a) carbinolamine b) hydrazone c) imine d) hydrazine e) aminalarrow_forwardAmide hydrolysis in basic conditions forms A. a carboxylic acid and an amine B. a carboxylate salt and an amine 3. an ester and an amine 4. a carboxylic acid and an amine saltarrow_forward
- When two equivalents of an amine are treated with acetyl chloride, both equivalents of the amine attack the carbonyl group, to give the product shown. 2 O True O False NH₂ CI NH ANH HO + HCIarrow_forwardA. The water solubility of amines found in medications can be increased by salt formation. Dextromethorphan hydrobromide, a cough suppresant, is the salt formed from dextromethorphan (XX) and hydrobromic acid (HBr). Write the equation for the formation of dextromethorphan hydrobromide. B. Suggest appropriate starting materials ( amine and alkyl halide) needed for the preparation of each of the following salts: 1. Cetylpyridinium chloride 2. Benzyldimethyltetradecylammonium chloridearrow_forwardShow how to synthesize the following amines from the indicated starting materials.(a) N-cyclopentylaniline from aniline (b) N-ethylpyrrolidine from pyrrolidinearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning