
Concept explainers
(a)
Interpretation:
To draw the structural formula of given organic compounds.
Concept Introduction:
Ester, amide and anhydride are derivatives of carboxylic acid.
Answer to Problem 19.4P
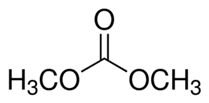
Explanation of Solution
We know that
(b)
Interpretation:
To draw the structural formula of given organic compounds.
Concept Introduction:
Ester, amide and anhydride are derivatives of carboxylic acid.
Answer to Problem 19.4P
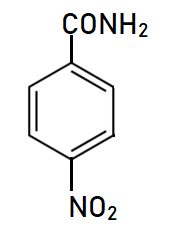
Explanation of Solution
This is a nitro derivative of benzamide in which nitro group is bonded at para position with respect to amide group. Benzamide is
(c)
Interpretation:
To draw the structural formula of given organic compounds.
Concept Introduction:
Ester, amide and anhydride are derivatives of carboxylic acid.
Answer to Problem 19.4P
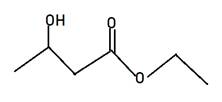
Explanation of Solution
Alkyl alkanoate is the general name for ester. Here Alkyl indicates the small alkyl group in RCOOR and alkanoate is RCOO- part. The name given is ethyl 3-hydroxybutanate, hence 3-hydroxybutanate will be RCOO-part and ethyl will be another r of ester. Therefore, the structure of ethyl 3-hydroxybutanate will be
(d)
Interpretation:
To draw the structural formula of given organic compounds.
Concept Introduction:
Ester, amide and anhydride are derivatives of carboxylic acid.
Answer to Problem 19.4P
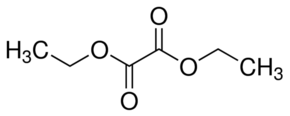
Explanation of Solution
Diethyl oxalate is the diester of oxalic acid (HOOC-COOH). As name suggested, diethyl stands for two ethyl group bonded at both carbon atoms of oxalic acid hence the formula will be
(e)
Interpretation:
To draw the structural formula of given organic compounds.
Concept Introduction:
Ester, amide and anhydride are derivatives of carboxylic acid.
Answer to Problem 19.4P
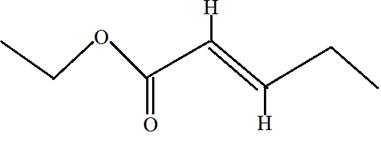
Ethyl trans-2-pentenoate.
Explanation of Solution
Ethyl trans-2-pentenoate is the ester of 2-pentenoic acid with ethanol. Here trans indicates the position of H on both double bonded carbon atoms. Hence in the formula of ester R1 -COO-R2 ; R2 will be ethyl group from alcohol and R1 will be alkyl group from acid.
(f)
Interpretation:
To draw the structural formula of given organic compounds.
Concept Introduction:
Ester, amide and anhydride are derivatives of carboxylic acid.
Answer to Problem 19.4P
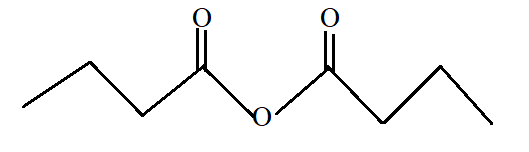
Butanoic anhydride.
Explanation of Solution
Butanoic anhydride is the anhydride of butanoic acid with −COOCO- as functional group. Butanoic acid is
Want to see more full solutions like this?
Chapter 19 Solutions
Introduction to General, Organic and Biochemistry
- 2. What is the organic by-product in a haloform reaction? A) an ether B) a haloform C) an alcohol D) an ester E) an amidearrow_forwardGive the expected organic product when phenylacetic acid, PhCH2COOH, is treated with reagent Q.)NaOH, H2Oarrow_forwardGive the expected organic product when phenylacetic acid, PhCH2COOH, is treated with reagent Q.)NaHCO3, H2Oarrow_forward
- Treatment of ethyl acetoacetate with NaOEt (2 equiv) and BrCH2CH2Br forms compound X. This reaction is the rst step in the synthesis of illudin-S, an antitumor substance isolated from the jack-o’-lantern, a poisonous, saffron-colored mushroom. What is the structure of X?arrow_forwardPredict the chemical name of compound B a. p-chlorobenzylamine b. 4-bromoaniline c. benzylamine d. p-chloronitrile e. p-chlorobenzaldehyde 2. What is the reaction name for the chemical transformation of A to B a. reductive amination b. catalytic reduction c. carbonyl dehydration d. Hofmann elimination e. Aldehyde rearrangementarrow_forwardThe oxidation of 3-methylbutanal with potassium permanganate in an acid medium produces compound A and the reduction of cyclohexanone with NaBH4 produces compound B. Indicate the CORRECT alternative: a) A reacts rapidly with water to produce a carboxylic acid. b) Reaction of B with methylamine produces an amide. c) Reaction of A with an acyl chloride produces an ester. d) B reacts with HCN to produce a cyanohydrin. e) The reaction of A and B in an acid medium produces cyclohexyl 3-methylbutanoate.arrow_forward
- Draw a structural formula for the product of the reaction of acetophenone with reagent Q. N2H4, KOH at 250°Carrow_forward1. Which is more reactive? a. carboxylic acid b. acid anhydride c. acid halide d. amides e. esters 2. Removal of water? a. carboxylic acid b. acid anhydride c. acid halide d. amides e. esters 3. Condensation of alcohol and RCOOH? a. carboxylic acid b. acid anhydride c. acid halide d. amides e. estersarrow_forwardWhat carboxylic acid and amine are needed to synthesize the pain reliever phenacetin? Phenacetin was once a component of the over-the-counter pain reliever APC (aspirin, phenacetin, caffeine), but it is no longer used because of its kidney toxicity.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

