
a)
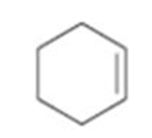
Interpretation:
How to prepare cyclohexene from 2-cyclohexenone is to be stated.
Concept introduction:
Wolf-Kishner reduction rection can be used to prepare cyclohexene from 2-cyclohexenone.
To state:
How to prepare cyclohexene from 2-cyclohexenone.
b)
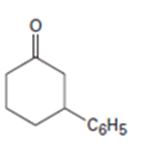
Interpretation:
How to prepare 3-phenylcyclohexanone from 2-cyclohexenone is to be stated.
Concept introduction:
By treating 2-cyclohexenone with lithiumdiphenylcopper and acidifying the product formed 3-phenylcyclohexanone can be prepared, as the organolithiumcopper reagents are good reagents for the conjugate addition to α,β- unsaturated
To state:
How to prepare 3-phenylcyclohexanone from 2-cyclohexenone.
c)
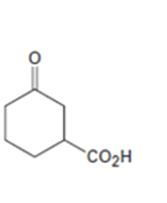
Interpretation:
How to prepare the ketoacid shown from 2-cyclohexenone is to be stated.
Concept introduction:
An easily oxidizable group is introduced to C3 of 2-cyclohexenone by a conjugate addition using lithiumdialkylcopper reagent. The resulting product can then be oxidized to a carboxylic acid by acidified KMnO4.
To state:
How to prepare the keto acid shown from 2-cyclohexenone.
d)
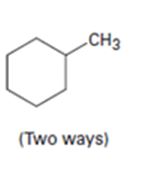
Interpretation:
How to prepare methylcyclohexane from 2-cyclohexenone (two ways) is to be shown.
Concept introduction:
An alkyl group can be introduced into 2-cyclohexenone by treating it with lithiumdialkyl reagent(conjugate addition takes place). The keto group can be reduced to CH2 by Wolff-Kishner reduction.
Another way is to replace the C =O in 2-cyclohexenone by C =CH2 by a Wittig reaction first and then reducing it to C-CH3 by H2, Pd/C.
To state:
How to prepare methylcyclohexane from 2-cyclohexenone.
Trending nowThis is a popular solution!

Chapter 19 Solutions
Organic Chemistry
- What product do you expect from the reaction of the following etherswith HBr? a. CH3CH2OCH2CH3 b. cyclohexyl ethyl etherarrow_forwardHow could you prepare 3,3-dibromohexane from reagents that contain no more than two carbons?arrow_forwardStarting with cyclohexane, how could the following compounds be prepared?arrow_forward
- How would you prepare the following compounds from 2-phenylethanol? More than one step may be required.arrow_forwardShow how could you prepare the following compounds starting from benzene:arrow_forwardHow can you prepare the following compounds with benzene as one of the starting materials?arrow_forward
