
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
9th Edition
ISBN: 9780136781776
Author: Wade
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 20.34SP
Given the structure of ascorbic acid (vitamin C):
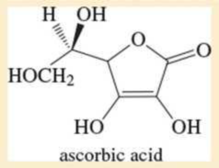
- a. Is ascorbic acid a carboxylic acid?
- b. Compare the acid strength of ascorbic acid (pKa = 4.71) with acetic acid.
- c. Predict which proton in ascorbic acid is the most acidic.
- d. Draw the form of ascorbic acid that is present in the body (aqueous solution, pH = 7.4).
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A – if both statements are correct.B – if the first statement is correct and the second is wrong C – if the first statement is wrong and the second is correct.D – if both statements are wrong.
Draw the products of each acid–base reaction.
Draw the organic product for A, B, and C
Chapter 20 Solutions
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
Ch. 20.2C - Prob. 20.1PCh. 20.2C - Name the following carboxylic acids (when...Ch. 20.4B - Rank the compounds in each set in order of...Ch. 20.5 - Prob. 20.4PCh. 20.5 - Phenols are less acidic than carboxylic acids,...Ch. 20.5 - Prob. 20.6PCh. 20.7A - Prob. 20.7PCh. 20.7B - Prob. 20.8PCh. 20.7D - Draw all four resonance forms of the fragment at...Ch. 20.7D - a. Why do most long-chain fatty acids show a large...
Ch. 20.10 - Prob. 20.13PCh. 20.10 - A carboxylic acid has two oxygen atoms, each with...Ch. 20.10 - Prob. 20.15PCh. 20.10 - The mechanism of the Fischer esterification was...Ch. 20.10 - Prob. 20.17PCh. 20.12 - Show how to synthesize the following compounds,...Ch. 20.13 - Show how you would synthesize the following...Ch. 20.14 - Prob. 20.20PCh. 20.14 - Prob. 20.21PCh. 20.15 - Propose a mechanism for the reaction of benzoic...Ch. 20.15 - Prob. 20.23PCh. 20.15 - Prob. 20.24PCh. 20 - Prob. 20.25SPCh. 20 - Give both IUPAC names and common names for the...Ch. 20 - Draw the structures of the following compounds. a....Ch. 20 - Prob. 20.28SPCh. 20 - Arrange each group of compounds in order of...Ch. 20 - Predict the products (if any) of the following...Ch. 20 - Rank the following isomers in order of increasing...Ch. 20 - Prob. 20.32SPCh. 20 - What do the following pKa values tell you about...Ch. 20 - Given the structure of ascorbic acid (vitamin C):...Ch. 20 - Prob. 20.35SPCh. 20 - Show how you would accomplish the following...Ch. 20 - Predict the products and propose mechanisms for...Ch. 20 - Prob. 20.38SPCh. 20 - Prob. 20.39SPCh. 20 - Prob. 20.40SPCh. 20 - Prob. 20.44SPCh. 20 - Prob. 20.45SPCh. 20 - Predict the major form of each compound when it is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- This reaction makes a carboxylic acid, draw the reaction peoducts for both 1 and 2 bazaupalb an aolu DA 1. KMnO4, NaOH, H₂O, heat 2. H₂O, H3O+ catalystarrow_forwardAmino acids such as glycine are the building blocks of large molecules called proteins that give structure to muscle, tendon, hair, and nails. a. Explain why glycine does not actually exist in the form with all atoms uncharged, but actually exists as a salt called a zwitterion. b. What product is formed when glycine is treated with concentrated HCl? c. What product is formed when glycine is treated with NaOH?arrow_forwardDraw the products of each acid-base reaction. NaOH b. + NazCO3 a. + CH3CH,CH, HO, OHarrow_forward
- The Ka1 of ascorbic acid is 7.94 x 10-5. Would you expect ascorbic acid dissolved in blood plasma (pH 7.35–7.45) to exist primarily as ascorbic acid or as ascorbate anion? Explain.arrow_forwardDraw the following reactions and label it properly 1. stearic acid reaction with water chemical equation 2. Reaction with NaOH with benzoic acid 3. Reaction with NaOH with acetic acid 4. Reaction with NaOH with stearic acid 5. Reaction with sodium carbonate with acetic acidarrow_forwardDraw the products of each Lewis acid–base reaction. Label the electrophile and nucleophile.arrow_forward
- Which member of each pair is the stronger base? a. ethylamine or aniline b. ethylamine or ethoxide ion c. phenolate ion or ethoxide ion d. phenolate ion or acetate ionarrow_forwardRank the compound of the attached group from strongest acid to weakest acid:arrow_forwardWhat happens when a carboxylic acid is treated with 1.PBr3 (or P),Br. 2.H2Oarrow_forward
- Consider the structure below. (1) Draw its conjugate base. (2) Draw its conjugate acid. H₂N- -OHarrow_forwardHow can the structure of the following molecule be changed in order to make it more acidic? A. Replacing the CI substituent with OH 8. Moving the CI substituent to the C position C. Moving the CI substituent to the C position D. Replacing the CI substituent with an NH₂ явон The most common reaction involving the carboxylic acids and its derivatives is related to the presence of an acyl group. In the structure shown below, which group corresponds to the alkyl group of the acyl structure? A. n-propyl group 8. Chloro group C. Isobutyl group D. Carbonyl group An aldehyde commonly exhibits a nucleophilic addition type of reaction. When a nucleophile attacks a carbonyl carbon, what happens to the oxygen atom in the structure? Refer to the structure below. A. Oxygen atom obtains a net negative charge. B. Oxygen atom becomes more electronegative. C. Oxygen atom acts as the new electrophile. D. Oxygen atom transforms to an alkoxide group.arrow_forwardDraw the possible products of this epoxide ring-opening reaction. Use a dash or wedge bond to indicate the stereochemistry of substituents on asymmetric centers, where applicableIgnore any inorganic byproducts. 41100 H 1) NaOH / H₂O 2) dilute HCI ☑Ⓒ Please select a drawing or reagent from the question areaarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY