
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
9th Edition
ISBN: 9780136781776
Author: Wade
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 20.46SP
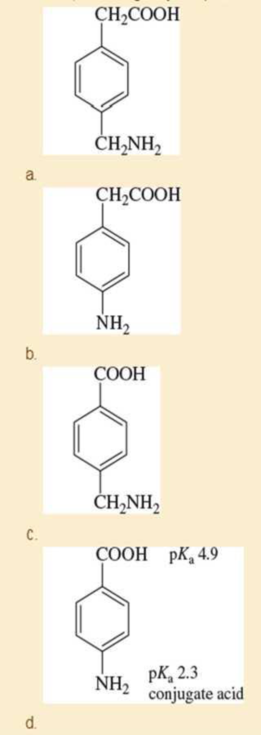
Predict the major form of each compound when it is dissolved in pure water. To do this, you will need to estimate pKa values for the compounds in (a), (b), and (c) based on similar compounds shown in Appendix 4. Values for the compound in (d) are given. Explain the differences, including why the pKa values in part (d) are so different from the others.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The pka for 2-hydroxybenzoic acid (i.e. salicylic acid, used in pain relief and a precursor
for aspirin) is 2.98, while the pKa for 3-hydroxybenzoic acid (found in castoreum, a scent-
marking substance secreted by beavers) is 4.08. What does this difference in pK₂ tell us
about the relative acidities of these two compounds?, Why is there such a significant
difference?
OH
OH
2-hydroxybenzoic acid (pKa = 2.98)
OH
OH
3-hydroxybenzoic acid (pKa 4.08)
What do you mean by carbon acid? What is the range of their pKa's?
Aniline (conjugate acid pKa 4.63) is a considerably stronger base than diphenylamine (pKa 0.79). Account for these marked differences.
Chapter 20 Solutions
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
Ch. 20.2C - Prob. 20.1PCh. 20.2C - Name the following carboxylic acids (when...Ch. 20.4B - Rank the compounds in each set in order of...Ch. 20.5 - Prob. 20.4PCh. 20.5 - Phenols are less acidic than carboxylic acids,...Ch. 20.5 - Prob. 20.6PCh. 20.7A - Prob. 20.7PCh. 20.7B - Prob. 20.8PCh. 20.7D - Draw all four resonance forms of the fragment at...Ch. 20.7D - a. Why do most long-chain fatty acids show a large...
Ch. 20.10 - Prob. 20.13PCh. 20.10 - A carboxylic acid has two oxygen atoms, each with...Ch. 20.10 - Prob. 20.15PCh. 20.10 - The mechanism of the Fischer esterification was...Ch. 20.10 - Prob. 20.17PCh. 20.12 - Show how to synthesize the following compounds,...Ch. 20.13 - Show how you would synthesize the following...Ch. 20.14 - Prob. 20.20PCh. 20.14 - Prob. 20.21PCh. 20.15 - Propose a mechanism for the reaction of benzoic...Ch. 20.15 - Prob. 20.23PCh. 20.15 - Prob. 20.24PCh. 20 - Prob. 20.25SPCh. 20 - Give both IUPAC names and common names for the...Ch. 20 - Draw the structures of the following compounds. a....Ch. 20 - Prob. 20.28SPCh. 20 - Arrange each group of compounds in order of...Ch. 20 - Predict the products (if any) of the following...Ch. 20 - Rank the following isomers in order of increasing...Ch. 20 - Prob. 20.32SPCh. 20 - What do the following pKa values tell you about...Ch. 20 - Given the structure of ascorbic acid (vitamin C):...Ch. 20 - Prob. 20.35SPCh. 20 - Show how you would accomplish the following...Ch. 20 - Predict the products and propose mechanisms for...Ch. 20 - Prob. 20.38SPCh. 20 - Prob. 20.39SPCh. 20 - Prob. 20.40SPCh. 20 - Prob. 20.44SPCh. 20 - Prob. 20.45SPCh. 20 - Predict the major form of each compound when it is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Suppose you have a mixture of these three compounds. Devise a chemical procedure based on their relative acidity or basicity to separate and isolate each in pure form.arrow_forwardWhich is a stronger acid and has the most stable conjugate base? Estimate the pKA values for both compounds.arrow_forwardCompounds like amphetamine that contain nitrogen atoms are protonated by the HCl in the gastric juices of the stomach, and the resulting salt is then deprotonated in the basic environment of the intestines to regenerate the neutral form. Write proton transfer reactions for both of these processes.arrow_forward
- Arrange the compounds in each set in order of increasing acid strength. consult Table 4.1 for pKa values of each acid.arrow_forward3. You are given a mixture of aspirin, phenol, and naphthalene that you need to separate. i) Draw the structures of each, and identify if they are acidic, basic, or neutral compounds. For each compound draw their reaction with the appropriate acidic or basic conditions that will change their solubility and allow them to be separated. ii) What modifications would you have to make to the experimental protocol in order to separate these three compounds? Provide specifics.arrow_forward3) Consider the molecules of but-2-enal and but-3-enal below. Both molecules can be deprotonated by a strong base such as lithium diisopropylamide (LDA). H 2 H But-3-enal But-2-enal Which molecule is more acidic? Why? Use a reaction coordinate diagram(s) to explain your answer.arrow_forward
- Pyridine forms a stronger Lewis acid-base complex with SO3 than SO2. However, pyridine forms a weaker complex with SF6 than with SF4. Explain the difference.arrow_forwardPhthalic acid and isophthalic acid have protons on two carboxy groups that can be removed with base. (a) Explain why the pKa for loss of the rst proton (pKa1) is lower for phthalic acid than isophthalic acid. (b) Explain why the pKa for loss of the second proton (pKa2) is higher for phthalic acid than isophthalic acid.arrow_forwardPhthalic acid and isophthalic acid have protons on two carboxy groups that can be removed with base. (a) Explain why the pKa for loss of the first proton (pKa1) is lower for phthalic acid than isophthalic acid. (b) Explain why the pKa for loss of the second proton (pKa2) is higher for phthalic acid than isophthalic acid.arrow_forward
- Phthalic acid and isophthalic acid have protons on two carboxy groupsthat can be removed with base. (a) Explain why the pKa for loss of thefirst proton (pKa1) is lower for phthalic acid than isophthalic acid. (b)Explain why the pKa for loss of the second proton (pKa2) is higher forphthalic acid than isophthalic acid.arrow_forwardThe weakest of the acids below is and its pK is The strongest of the acids below is and its pK is 4.21 4.76 imidazolium ion 5.64 8.20 succinate boric acid formic acid 4.21 4.76 imidazolium ion 5.64 8.20 succinate boric acid formic acid phosphoric acid succinic acid 3.75 7.00 p-nitrophenol 9.24 acetic acid 0.18 9.25 glycinamide ammonium ion 2.15 7.24arrow_forward8) Which compound is more acidic: 1) Aminomethan or aniline? 2) Ethanol or phenol? Draw the mesomeric structures of the acids and their conjugate bases to explain their relative stability.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY