
Concept explainers
(a)
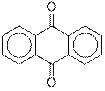
Interpretation: The given compound anthraquinone needs to be identified as an
Concept Introduction : Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
| Aldehyde | 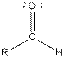 |
| Ketone | 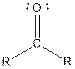 |
| Carboxylic acid | 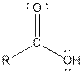 |
| Ester | 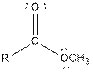 |
| Amine | 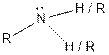 |
(a)
Answer to Problem 37E
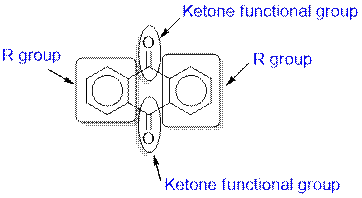
Anthraquinone contains a ketone functional group.
Explanation of Solution
From the table, the general structure for ketone is as follows:
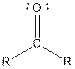
The given compound contains a ketone functional group shown as:
(b)
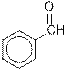
Interpretation: The given compounds needs to be identified as an aldehyde, ester, carboxylic acid, ketone or amine.
Concept Introduction : Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
| Aldehyde | 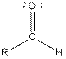 |
| Ketone | 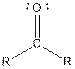 |
| Carboxylic acid | 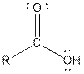 |
| Ester | 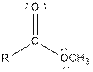 |
| Amine | 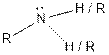 |
(b)
Answer to Problem 37E
The given organic compound contains an aldehyde functional group.
Explanation of Solution
From the table, the general structure for an aldehyde is as follows:
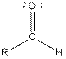
The given compound contains an aldehyde functional group shown as:

(c)
Interpretation: The given compounds needs to be identified as an aldehyde, ester, carboxylic acid, ketone or amine.
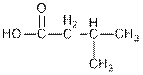
Concept Introduction : Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
| Aldehyde | 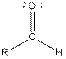 |
| Ketone | 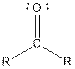 |
| Carboxylic acid | 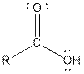 |
| Ester | 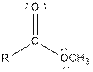 |
| Amine | 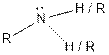 |
(c)
Answer to Problem 37E
The given organic compound contains a carboxylic acid functional group.
Explanation of Solution
From the table, the general structure for carboxylic acid functional group is as follows:
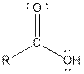
The given compound contains a carboxylic acid functional group shown as:
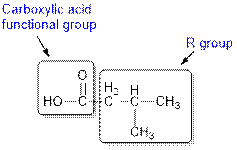
(d)
Interpretation: The given compounds needs to be identified as an aldehyde, ester, carboxylic acid, ketone or amine.
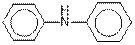
Concept Introduction: Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
| Aldehyde | 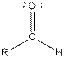 |
| Ketone | 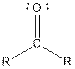 |
| Carboxylic acid | 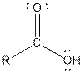 |
| Ester | 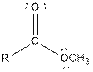 |
| Amine | 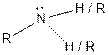 |
(d)
Answer to Problem 37E
The given organic compound contains an amine functional group.
Explanation of Solution
From the table, the general structure for amine functional group is as follows:
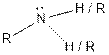
The given compound contains a carboxylic acid functional group shown as:
Want to see more full solutions like this?
Chapter 21 Solutions
CHEM.PRINC.W/OWL2+REBATE+2 SUPPL.>IP<
- Fatty acids are carboxylic acids that have long hydrocarbon chains attached to a carboxylate group. How does a saturated fatty acid differ from an unsaturated fatty acid? How are they similar?arrow_forwardExplain what is the difference between a terpene and a terpenoid. Illustrate your answer (drawstructures) with an examplearrow_forwardacetylsalicylic acid vs salicylic acid? What is the main chemical difference? Why can acetylsalicylic acid pass through the stomach without harm while salicylic acid can cause harm to the stomach????arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





