
Concept explainers
(a)
Interpretation: The functional groups present in the given compound needs to be identified.
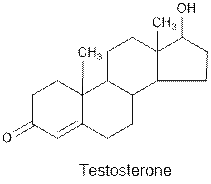
Concept Introduction : Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
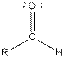 | |
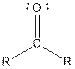 | |
| Carboxylic acid | 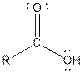 |
| Ester | 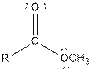 |
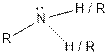 | |
| Alcohol | 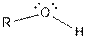 |
(a)
Answer to Problem 38E
Testosterone contains ketone and alcohol functional group.
Explanation of Solution
The general structure for ketone and alcohol are as follows:
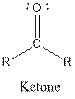
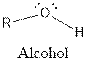
Hence, ketone and alcohol functional groups are present in the given compound as:
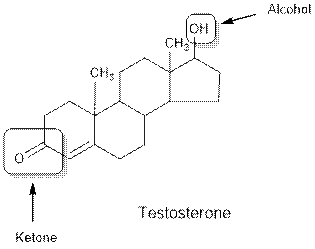
(b)
Interpretation: The functional groups present in the given compound needs to be determined.
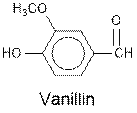
Concept Introduction : Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
| Aldehyde | 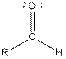 |
| Ketone | 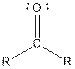 |
| Carboxylic acid | 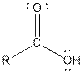 |
| Ester | 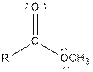 |
| Amine | 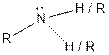 |
| Ether | 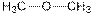 |
(b)
Answer to Problem 38E
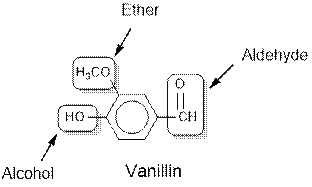
Vanillin contains alcohol, ether and aldehyde functional groups.
Explanation of Solution
The general structure for ether, aldehyde and alcohol are as follows:
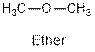
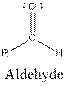
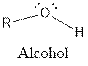
Hence, ether, aldehyde and alcohol functional groups are present in the given compound as:
(c)
Interpretation: The functional groups present in the given compound needs to be determined.
Concept Introduction : Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
| Aldehyde | 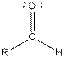 |
| Ketone | 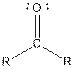 |
| Carboxylic acid | 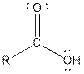 |
| Ester | 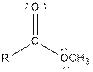 |
| Amine | 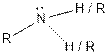 |
| Amide | 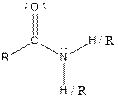 |
(c)
Answer to Problem 38E
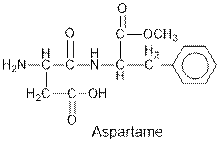
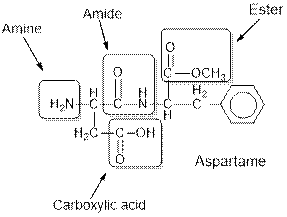
Aspartame contains carboxylic acid, amine, amide and ester functional groups.
Explanation of Solution
The general structure for ether, aldehyde and alcohol are as follows:
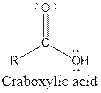

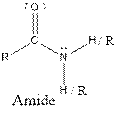
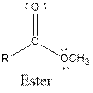
Hence, carboxylic acid, amine, amide and ester functional groups are present in the given compound as:
Want to see more full solutions like this?
Chapter 21 Solutions
CHEM.PRINC.W/OWL2+REBATE+2 SUPPL.>IP<
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





