
Concept explainers
Interpretation:
The general structure of amino acid with side chain and peptide bond needs to be explained.
Concept introduction:
Proteins are composed of amino acids. The amino acid is consist of carboxylic acid and amino group and attached with a side chain as an R group.
The two amino acids are bonded together with the help of a peptide bond.
Answer to Problem 3RQ
- The side chain group of an amino acid is mention as (R).
- The peptide bond formed between two amino acids with the removal of the water molecule.
Explanation of Solution
The general structure of an amino acid is composed of at least one carboxylic group and one amino group and the attached side chain. The general structure of the amino acid is given as
The (R) group is represented as the side chain of the amino acid.
The peptide bond formation is shown in the following structure-
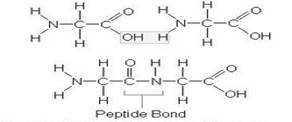
From the above stricture, water molecule is removed from two glycine amino acids to form a peptide bond.
- The general structure of amino acid with a side chain (R).
- The peptide bond formation with the removal of water molecule given as -
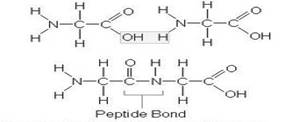
From the above stricture, water molecule is removed from two glycine amino acids to form a peptide bond.
Chapter 21 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





