
Concept explainers
Interpretation:
Lock and key model of the enzymes needs to be explained.
Concept introduction:
The biological catalyst is termed as enzymes. They are made up of different types of amino acids. They affect the speed of the biochemical reaction.
For the explanation of the action of the enzymes in the biochemical reaction, a famous theory is used that is the lock and key method.
Answer to Problem 7RQ
For the explanation of the catalytic nature of enzymes, a well know- method is the lock and key method. There are following two steps for this method-
Enzyme (E) + Substrate (S) = Enzyme Substrate Complex (ES)
Enzyme Substrate Complex (ES) = Enzyme (E) + Product (P).
Explanation of Solution
In the lock and key model the enzymes's activity shown by this diagram-
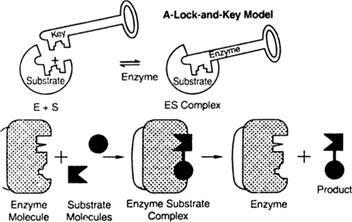
The enzymes attached to the substrate and formed the ES as enzymes substrate, the enzymes having the active site for the binding of the substrate just like a particular lock have a fixed key. A particular enzyme is fixed for a substrate. In the last step, the ES is converted into product and enzymes remain the same as staring at the reaction.
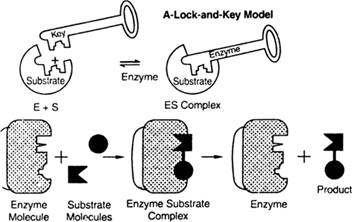
From the above diagram, the substrate is attached to a particularly active site of the enzyme for the formation of the product.
Chapter 21 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





