
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.17C, Problem 2.26P
Draw a Lewis structure and classify each of the following compounds.
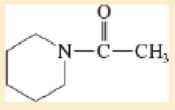
- a. CH3CH2CONHCH3
- b. (CH3CH2)2NH
- c. (CH3)2CHCOOCH3
- d. CH3CHCHCOCl
- e. (CH3CH2)2O
- f. CH3CH2CH2CN
- g. (CH3)3CCH2CH2COOH
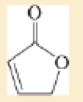
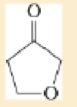
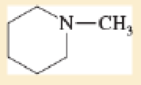
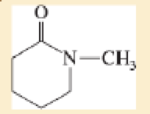
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using BEST Lewis structures and the VSEPR model, match each molecular formula with the molecular geometry that describes it.
In C=C, there are ____ atoms bonded to each other. The geometry is ______. In single bond will have_____ bonded atoms for each carbon. The geometry is now________
1.1 Define electronegativity and explain why electronegativity increases with atomic number within a period of the Periodic Table.
1.2 Describe the type of bonding that exists in F2 (g) molecule. How does this type of bonding differ from that found in the HF (g) molecule?
1.3 Define hydrogen bonding using examples.
1.4 PH3 and NH3 moleculea are the same shape but the molecules are non-polar and polar respectively. Explain why this is so.
1.5 Give an example of molecule that has a coordinate covalent bond.
1.6 Draw a Lewis structure for each of the following molecules of ions:
a) HF3
b) CIO3-
Chapter 2 Solutions
Organic Chemistry (9th Edition)
Ch. 2.1A - Prob. 2.1PCh. 2.1B - The NF bond is more polar than the NH bond: but...Ch. 2.1B - For each of the following compounds 1. Draw the...Ch. 2.1B - Two isomers of 1,2-dichloroethene are known One...Ch. 2.2C - Prob. 2.5PCh. 2.2C - Prob. 2.6PCh. 2.3 - Prob. 2.7PCh. 2.4 - Calculate the pH of the following solutions a....Ch. 2.6A - Ammonia appears in Table 2-2 as both an acid and a...Ch. 2.7 - Write equations for the following acid-base...
Ch. 2.7 - Ethanol, methylamine. and acetic acid are all...Ch. 2.8 - Prob. 2.12PCh. 2.10 - Write equations for the following acid-base...Ch. 2.10 - Rank the following acids in decreasing order of...Ch. 2.11 - Prob. 2.15PCh. 2.11 - Prob. 2.16PCh. 2.11 - Consider each pair of bases and explain which one...Ch. 2.12 - Which is a stronger base ethoxide ion or acetate...Ch. 2.12 - Prob. 2.19PCh. 2.12 - Prob. 2.20PCh. 2.12 - Prob. 2.21PCh. 2.12 - Choose the more basic member of each pair of...Ch. 2.14 - Prob. 2.23PCh. 2.15D - Classify the following hydrocarbons and draw a...Ch. 2.16D - Prob. 2.25PCh. 2.17C - Draw a Lewis structure and classify each of the...Ch. 2.17C - Circle the functional groups in the following...Ch. 2 - The CN triple bond in acetonitrile has a dipole...Ch. 2 - Prob. 2.29SPCh. 2 - Sulfur dioxide has a dipole moment of 1.60 D....Ch. 2 - Which of the following pure compounds can form...Ch. 2 - Predict which member of each pair is more soluble...Ch. 2 - Prob. 2.33SPCh. 2 - Prob. 2.34SPCh. 2 - Predict which compound in each pair has the higher...Ch. 2 - All of the following compounds can react as acids...Ch. 2 - Rank the following species in order of increasing...Ch. 2 - Rank the following species in order of increasing...Ch. 2 - The Ka of phenylacetic acid is 5 2 105, and the...Ch. 2 - The following compound can become protonated on...Ch. 2 - The following compounds are listed in increasing...Ch. 2 - Prob. 2.42SPCh. 2 - Prob. 2.43SPCh. 2 - Compare the relative acidity of 1-molar aqueous...Ch. 2 - The following compounds can all react as acids. a....Ch. 2 - The following compounds can all react as bases. a....Ch. 2 - The following compounds can all react as acids. a....Ch. 2 - Prob. 2.48SPCh. 2 - Methyllithium (CH3Li) is often used as a base in...Ch. 2 - Label the reactants in these acid-base reactions...Ch. 2 - In each reaction, label the reactants as Lewis...Ch. 2 - Prob. 2.52SPCh. 2 - Each of these compounds can react as a nucleophile...Ch. 2 - Prob. 2.54SPCh. 2 - Give a definition and an example for each class of...Ch. 2 - Circle the functional groups in the following...Ch. 2 - Prob. 2.57SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Atom A has 4 valence electrons. Atom Z has 6 valence electrons. For the AZ3-2 ion How many valence electrons are in the structure? b. How many single bonds are in the structure? c. How many double bonds are in the structure? d. How many triple bonds are in the structure? e. How many lone pairs are on the central atom in the structure? f. What is the shape of the structure? g. What are the bond angles of this ion?arrow_forwardWhy is the geometric structure of a molecule important, especially for biological molecules?arrow_forwardConsider the following flat drawing of methane (CH4) . a. What is HCH bond angle implied by this drawing if you assume it is flat? b. Are the electron domains of this flat CH4 spread out as much as possible? c. Use model materials to make a model of CH4 (methane). If you assembled it correctly, thefour bonds (bonding electron domains) of your model will be 109.5° apart. d. In which representation, the drawing above or the model in your hand (circle one) are theH’s of CH4 more spread out around the central carbon? e. Confirm that your model looks like the following drawing. The wedgebond represents a bond coming out of the page, and the dash bondrepresents a bond going into the page f. You will often see methane drawn as if it were flat (like on the previous page). Why is thismisleading, and what is left to the viewer’s imagination when looking at such a drawing?arrow_forward
- Part A) Consider the structural changes that occur in the following molecules. Begin by drawing the best Lewis Structure for each of the following molecules. BH3 CH4 NH3 H2O HF Part B)What are the ideal bond angles for each structure, and which are expected to be distorted? For the ones that are distorted look up on the internet and record their experimental values here: Part C)According to Lewis and VSEPR theory, why do these changes occur?arrow_forwarda) how many total pairs of electrons does CF4 have? b) How many bonded pairs and how many lone pairs does CF4 have? c) What is the molecular shape and angle of CF4? d) what is the lewis structure for CF4?arrow_forward- What orbital(s) are the lone pairs of electrons on the O atom placed? - What orbital is the lone pair of electrons on the N atom placed? - What type of bond(s) are formed between Ca1 – C? - What type of bond(s) are formed between C = O? - What type of bond(s) are formed between C – N? - What type of bond(s) are formed between N – Ca2? - What type of bond(s) are formed between N – H? - What is the angle between Ca1 – C = O? - What is the angle between Ca1 – C – N? - What is the angle between O = C – N? - What is the angle between C – N – Ca2?arrow_forward
- The trans arrangement is favored in N2H2, but the cis arrangement is favored in N2F2. Explain this result?arrow_forwardDraw the lewis structure of SO2 ( best resonance) and 2nd best resonance showing the shape and bond angles of each one, the 3D structure with polar bonds or bonds dipole of each one and the molecular polarity. Here is an example of what I want the answer to be like:arrow_forwardLewis structure of H2S. Any polar bonds in molecule? Polar or non polar?arrow_forward
- Propylene, C3H6,C3H6, is a gas that is used to form the important polymer called polypropylene. Its Lewis structure is (a) What is the total number of valence electrons in the propylene molecule? (b) How many valence electrons are used to make σσ bonds in the molecule? (c) How many valence electrons are used to make ππ bonds in the molecule? (d) How many valence electrons remain in nonbonding pairs in the molecule? (e) What is the hybridization at each carbon atom in the molecule?arrow_forwardA. Fluoromethane B. Methanol C. Chloromethane D. Water 1. Which molecule has the highest dipole moment? 2. Which molecule has the greatest bond angle relative to the electronegative atom? 3. Which molecule has the most optimal bond angle? 4. Which molecule is the most polar? 5. Which molecule contains the most electronegative atom?arrow_forwardWhich molecule has the shortest bond? N2 N2+ N22- N23- Which molecule has the highest bond enthalpy? N2 N2+ N22- N23- Which molecule has the weakest bond? N2 N2+ N22- N23-arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER


Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY