
(a)
Interpretation-
To draw the structural formula of the given compound.
Concept Introduction- The structural formula is the representation of a molecule that shows the arrangement of atoms and bonds.
Steps to draw the structural formula:
- Analyze the type of hydrocarbon using the suffix in the name.
- Start with drawing all the carbon-carbon bonds.
- Carbon can form four covalent bonds. Hence, complete the remaining bonds using hydrogen.
- Prefixes indicate the type of substituents and the times of each substituent. The number indicates the position of the substituent.
Cis- Trans Isomerism:
Cis-trans isomerism occurs when there is restricted rotation present and there are two non-identical groups attached to a double bond.
If the same group is present on the same side of the double bond, it is a cis isomer while if the same group is present on the opposite side of the double bond, it is trans.
(a)
Explanation of Solution
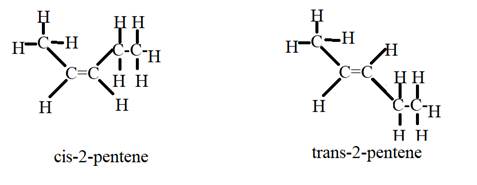
The given compound is
This has no substituents given in the prefix. The number two indicates that a double bond is present on the second carbon.
It can also show cis-trans isomerism.
Hence the structural formulas of
(b)
Interpretation-
To draw the structural formula of a given compound.
Concept Introduction- The structural formula is a representation of the molecule that shows the arrangement of atoms and bonds.
Steps to draw the structural formula:
- Analyze the type of hydrocarbon using the suffix in the name.
- Start with drawing all the carbon-carbon bonds.
- Carbon can form four covalent bonds. Hence complete the remaining bonds using hydrogen.
- Prefixes indicate the type of substituents and the times of each substituent. The number indicates the position of the substituent.
Cis- Trans Isomerism:
Cis-trans isomerism occurs when there is restricted rotation present and there are two non-identical groups attached to a double bond.
If the same group is present on the same side of the double bond, it is a cis isomer while if the same group is present on the opposite side of the double bond, it is trans.
(b)
Explanation of Solution
The given compound is
This has one substituent given in the prefix methyl at the second carbon atom of the parent chain. It has five carbons in the parent chain and the number two indicates that a double bond is present on the second carbon.
Hence, the structural formulas of
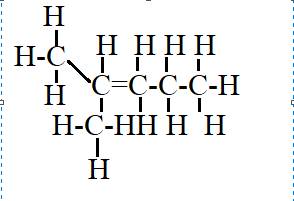
(c)
Interpretation-
To draw the structural formula of a given compound.
Concept Introduction- The structural formula is a representation of the molecule that shows the arrangement of atoms and bonds.
Steps to draw the structural formula:
- Analyze the type of hydrocarbon using the suffix in the name.
- Start with drawing all the carbon-carbon bonds.
- Carbon can form four covalent bonds. Hence complete the remaining bonds using hydrogen.
- Prefixes indicate the type of substituents and the times of each substituent. The number indicates the position of the substituent.
Cis- Trans Isomerism:
Cis-trans isomerism occurs when there is restricted rotation present and there are two non-identical groups attached to a double bond.
If the same group is present on the same side of the double bond, it is a cis isomer while if the same group is present on the opposite side of the double bond, it is trans.
(c)
Explanation of Solution
The given compound is
This has one substituent given in the prefix which is ethyl at the third carbon atom of the parent chain. It has five carbons in the parent chain and the number two indicates that a double bond is present on the second carbon.
Hence, the structural formulas of
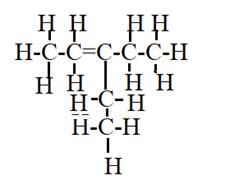
Chapter 22 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





