
(a)
Interpretation − To draw the structural formula of propyne.
Introduction −
Structural formula is representation of molecule that shows arrangement of atoms and bonds.
Steps to draw the structural formula:
- Analyse the type of hydrocarbon using suffix in name.
- Prefixes indicate the type of substituents and the times of each substituent. The number indicate the position of substituent
- Start with drawing all the carbon -carbon bonds.
- Carbon hydrogen bonds are understood.
(a)
Explanation of Solution
Propyne has three carbon atom chain and used the suffix “yne”, hence it is an
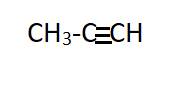
(b)
Interpretation − To draw the structure of cyclohexane,
Introduction −
Structural formula is representation of molecule that shows arrangement of atoms and bonds.
Steps to draw the structural formula:
- Analyse the type of hydrocarbon using suffix in name.
- Prefixes indicate the type of substituents and the times of each substituent. The number indicate the position of substituent
- Start with drawing all the carbon -carbon bonds.
- Carbon hydrogen bonds are understood.
(b)
Explanation of Solution
Cyclohexane is a cyclic structure with six carbon atoms containing the single bonds.
The structure can be:
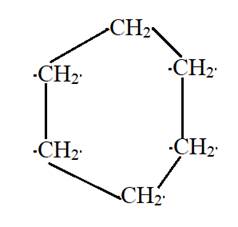
(c)
Interpretation − To draw the structure of
Introduction −
Structural formula is representation of molecule that shows arrangement of atoms and bonds.
Steps to draw the structural formula:
- Analyse the type of hydrocarbon using suffix in name.
- Prefixes indicate the type of substituents and the times of each substituent. The number indicate the position of substituent
- Start with drawing all the carbon -carbon bonds.
- Carbon hydrogen bonds are understood.
(c)
Explanation of Solution
The given compound is
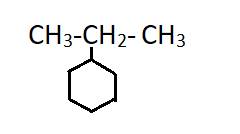
(d)
Interpretation − To draw the structure of
Introduction −
Structural formula is representation of molecule that shows arrangement of atoms and bonds.
Steps to draw the structural formula:
- Analyse the type of hydrocarbon using suffix in name.
- Prefixes indicate the type of substituents and the times of each substituent. The number indicate the position of substituent
- Start with drawing all the carbon -carbon bonds.
- Carbon hydrogen bonds are understood.
(d)
Explanation of Solution
The give compound
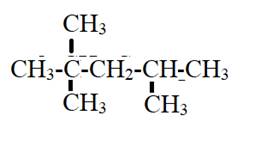
Chapter 22 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





