
Concept explainers
(a)
Interpretation:
The reagent and condition has to be proposed for step 1,2,3 and 5.
Concept introduction:
Hydrogenolysis:
Nitration: The formation of nitro group in a
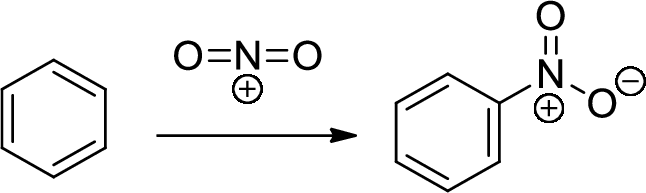
(b)
Interpretation:
The mechanism is to be proposed for iodination of 3-aminobenzoic acid.
Concept introduction:
Electrophilic
Halogenations on benzene:
Halogenation is one of the electrophilic substitution reactions. Halogens reaction with benzene (or electron donating group present in the benzene ring) which gives the corresponding halogenated compound.
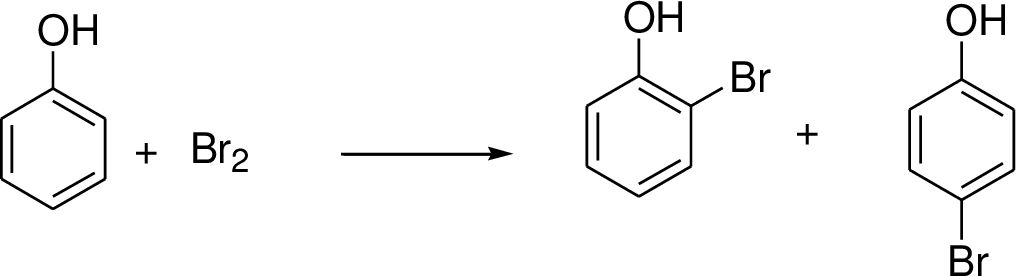
Trending nowThis is a popular solution!

Chapter 23 Solutions
Organic Chemistry
- A newer generation of antipsychotics, among them clozapine, are now used to treat the symptoms of schizophrenia. These drugs are more effective than earlier drugs in improving patient response in the areas of social withdrawal, apathy, memory, comprehension, and judgment. They also produce fewer side effects such as seizures and tardive dyskinesia (involuntary body movements). In the following synthesis of clozapine, Step 1 is an Ullmann coupling, a type of nucleophilic aromatic substitution that uses a copper catalyst. (a) Show how you might bring about formation of the amide in Step 2. (b) Propose a reagent for Step 3. (c) Propose a mechanism for Step 4. (d) Is clozapine chiral? If so, how many of the possible stereoisomers are formed in this synthesis?arrow_forwardSeveral sulfonylureas, a class of compounds containing RSO2NHCONHR, are useful drugs as orally active replacements for injected insulin in patients with adult-onset diabetes. These drugs decrease blood glucose concentrations by stimulating b cells of the pancreas to release insulin and by increasing the sensitivity of insulin receptors in peripheral tissues to insulin stimulation. Tolbutamide is synthesized by the reaction of the sodium salt of p-toluenesulfonamide and ethyl N-butylcarbamate . Propose a mechanism for this step.arrow_forwardAcid-catalyzed hydrolysis of HOCH2CH2C(CH3)2CN forms compound A (C6H10O2). A shows a strong peak in its IR spectrum at 1770 cm-1 and the following signals in its 1H NMR spectrum: 1.27 (singlet, 6 H), 2.12 (triplet, 2 H), and 4.26 (triplet, 2 H) ppm. Draw the structure for A and give a stepwise mechanism that accounts for its formation.arrow_forward
- 1. ) 2-Pentanol when introduced with H2SO4 will become ____________ . H2SO4 is a Dehydrating agent (used the concept of Zaitsev to come up with the major product)1-Pentene 1-Pentanal2-Pentene2- Pentanone2.) Diabetic breath has a mild sweet odor because of AroamticAldehydeAlkeneKetone3.) The following compounds contain a Carbonyl group, exceptAcetaminophenCitric AcidPhenolDacroonarrow_forwardWittig reactions with the following -chloroethers can be used for the synthesis of aldehydes and ketones. (a) Draw the structure of the triphenylphosphonium salt and Wittig reagent formed from each chloroether. (b) Draw the structural formula of the product formed by treating each Wittig reagent with cyclopentanone. Note that the functional group is an enol ether or, alternatively, a vinyl ether. (c) Draw the structural formula of the product formed on acid-catalyzed hydrolysis of each enol ether from part (b).arrow_forwardShow how to bring about each step in this synthesis of the herbicide propranil.arrow_forward
- Write the products of the following sequences of reactions. Refer to your reaction roadmaps to see how the combined reactions allow you to navigate between the different functional groups. Note that you will need your old Chapters 611, Chapters 1518, and Chapter 19 roadmaps along with your new Chapter 20 reaction roadmap for these.arrow_forwardUsing your reaction roadmap as a guide, show how to convert 1-bromopentane and sodium cyanide into N-hexylhexanamide. You must use 1-bromopentane and sodium cyanide as the source of all carbon atoms in the target molecule. Show all reagents and all molecules synthesized along the way.arrow_forwardWrite the products of the following sequences of reactions. Refer to your reaction roadmaps to see how the combined reactions allow you to navigate between the different functional groups. Note that you will need your old Chapters 611, Chapters 1518, and Chapter 19 roadmaps along with your new Chapters 2021 roadmaps for these.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
