
(a)
Interpretation:
Synthesis of given compound from benzene is to be shown.
Concept introduction:
In an electrophilic
Answer to Problem 23.80P
The given compound is synthesized from benzene as:
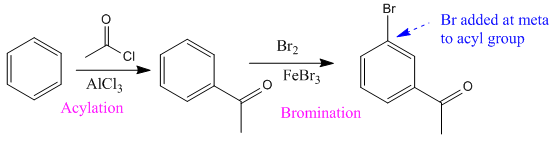
Explanation of Solution
The given compound is:
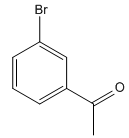
It is noticed that an electron-withdrawing carbonyl group is at meta-position to the bromine atom. Since the bromine atom (halogen) is an ortho/para directing group, acylation (addition of acyl group) of the benzene is carried out in the first step. The next step is the addition of chlorine, as the acyl group is meta directing incoming Cl added at meta to acyl group.
So the complete reaction of synthesis for the given compound is as:

Synthesis of the given compound from benzene is shown on the basis of the directing nature of substituent groups in an electrophilic aromatic substitution reaction.
(b)
Interpretation:
Synthesis of given compound from benzene is to be shown.
Concept introduction:
In an electrophilic aromatic substitution reaction, a substituent influences the site of reaction. The electron-donating groups are ortho/para directing groups, while electron-withdrawing groups are meta directing groups in the electrophilic aromatic substitution reaction.
Answer to Problem 23.80P
The given compound is synthesized from benzene as:
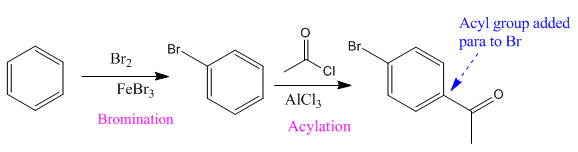
Explanation of Solution
The given compound is:
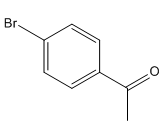
Here acyl group and Br are at para to each other, the Br group is an ortho/para director thus it added first to the benzene ring through bromination. The second step is acylation, the addition of acyl group at the para position.
So the complete reaction of synthesis for the given compound is as:

Synthesis of the given compound from benzene is shown on the basis of the directing nature of substituent groups in an electrophilic aromatic substitution reaction.
(c)
Interpretation:
Synthesis of given compound from benzene is to be shown.
Concept introduction:
In an electrophilic aromatic substitution reaction, a substituent influences the site of reaction. The electron-donating groups are ortho/para directing groups, while electron-withdrawing groups are meta directing groups in the electrophilic aromatic substitution reaction.
Answer to Problem 23.80P
The given compound is synthesized from benzene as:
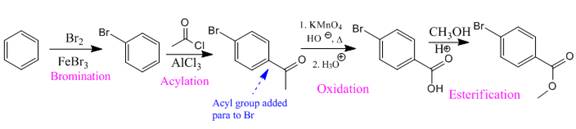
Explanation of Solution
The given compound is:
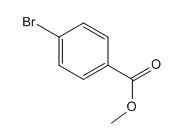
In the above compound both substituents are at para to each other, the Br group is an ortho/para director thus it added first to the benzene ring through bromination. The second step is acylation, addition of acyl group at the para position.

In the next step, the
So the complete reaction of synthesis for the given compound is as:
Synthesis of the given compound from benzene is shown on the basis of the directing nature of substituent groups in an electrophilic aromatic substitution reaction.
(d)
Interpretation:
Synthesis of given compound from benzene is to be shown.
Concept introduction:
In an electrophilic aromatic substitution reaction, a substituent influences the site of reaction. The electron-donating groups are ortho/para directing groups, while electron-withdrawing groups are meta directing groups in the electrophilic aromatic substitution reaction.
Answer to Problem 23.80P
The given compound is synthesized from benzene as:
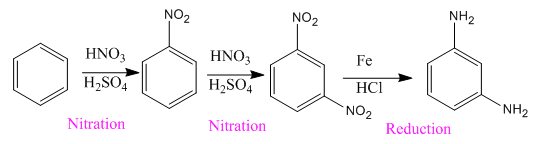
Explanation of Solution
The given compound is:
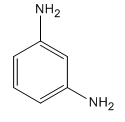
Here both substituents are amino
So the complete reaction of synthesis for the given compound is as:

Synthesis of the given compound from benzene is shown on the basis of the directing nature of substituent groups in an electrophilic aromatic substitution reaction.
(e)
Interpretation:
Synthesis of given compound from benzene is to be shown.
Concept introduction:
In an electrophilic aromatic substitution reaction, a substituent influences the site of reaction. The electron-donating groups are ortho/para directing groups, while electron-withdrawing groups are meta directing groups in the electrophilic aromatic substitution reaction.
Answer to Problem 23.80P
The given compound is synthesized from benzene as:
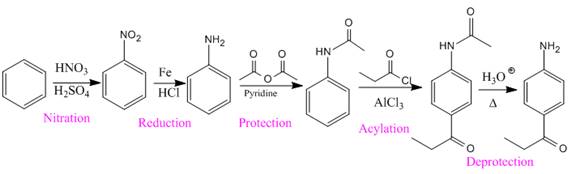
Explanation of Solution
The given compound is:
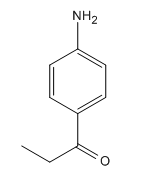
In the above compound, the amino and acyl groups are para to each other. The amino group is an ortho/para director and thus to be added first. The
So the complete reaction of synthesis for the given compound is as:

Synthesis of the given compound from benzene is shown on the basis of the directing nature of substituent groups in an electrophilic aromatic substitution reaction.
Want to see more full solutions like this?
Chapter 23 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





