
(a)
Interpretation: The products formed by the reaction of given compound with excess
Concept introduction:
Answer to Problem 25.62P
The products formed by the reaction of given compound with excess
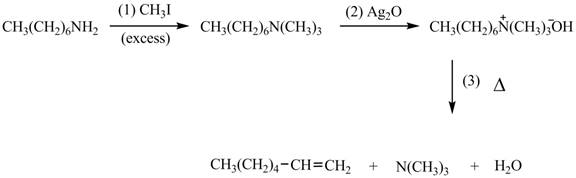
Explanation of Solution
Amines on reaction with excess
The products formed by the reaction of given compound with excess
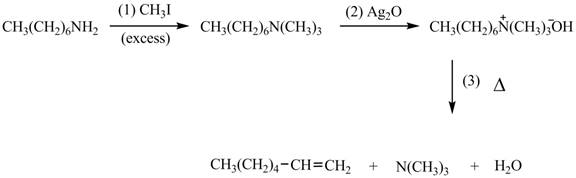
Figure 1
The products formed by the reaction of given compound with excess
(b)
Interpretation: The products formed by the reaction of given compound with excess
Concept introduction: Amines on reaction with excess
Answer to Problem 25.62P
The products formed by the reaction of given compound with excess
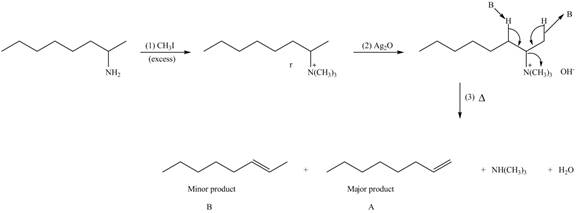
Explanation of Solution
Amines on reaction with excess
The products formed by the reaction of given compound with excess
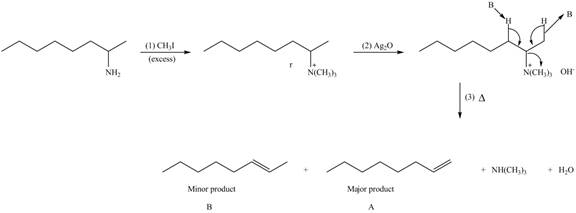
Figure 2
The products formed by the reaction of given compound with excess
(c)
Interpretation: The products formed by the reaction of given compound with excess
Concept introduction: Amines on reaction with excess
Answer to Problem 25.62P
The products formed by the reaction of given compound with excess
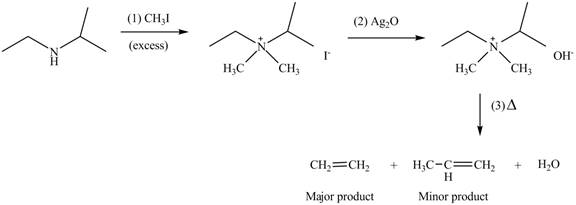
Explanation of Solution
Amines on reaction with excess
The products formed by the reaction of given compound with excess

Figure 3
The products formed by the reaction of given compound with excess
(d)
Interpretation: The products formed by the reaction of given compound with excess
Concept introduction: Amines on reaction with excess
Answer to Problem 25.62P
The products formed by the reaction of given compound with excess
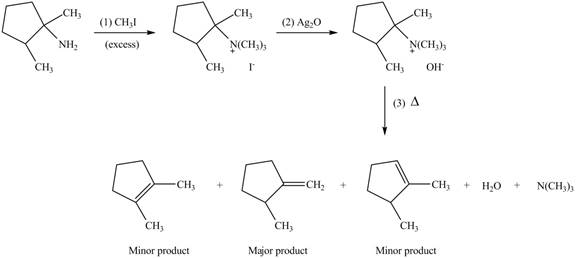
Explanation of Solution
Amines on reaction with excess
The products formed by the reaction of given compound with excess

Figure 4
The products formed by the reaction of given compound with excess
(e)
Interpretation: The products formed by the reaction of given compound with excess
Concept introduction: Amines on reaction with excess
Answer to Problem 25.62P
The products formed by the reaction of given compound with excess
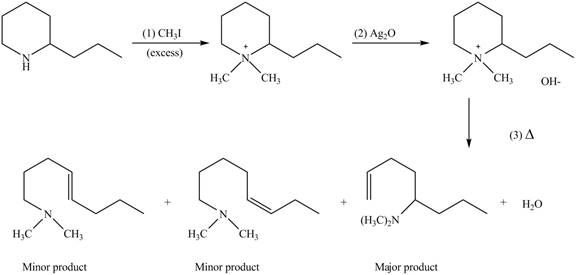
Explanation of Solution
Amines on reaction with excess
The products formed by the reaction of given compound with excess
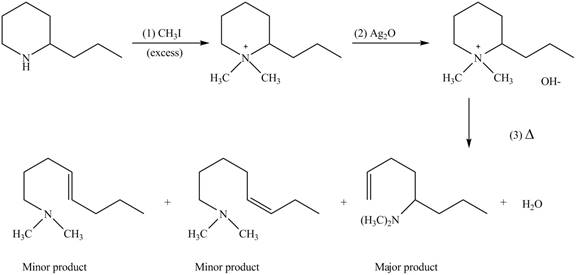
Figure 5
The products formed by the reaction of given compound with excess
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry -Study Guide / Solution Manual (Custom)
- Stanozolol is an anabolic steroid that promotes muscle growth. Althoughstanozolol has been used by athletes and body builders, many physicaland psychological problems result from prolonged use and it is bannedin competitive sports. Explain why the pKa of the N—H bond in the pyrazole ring iscomparable to the pKa of the O—H bond, making it considerably moreacidic than amines such as CH3NH2 (pKa = 40).arrow_forwardThe –NHCOR group of an amide is an activating group, but it is not as strongly activating as NH2. (a) Explain why it is an activating group. (b) Explain why it is less activating than NH2.arrow_forwardDraw the products formed when each carbonyl compound reacts with the following amines: [1] CH3CH2CH2NH2; [2] (CH3CH2)2NH.arrow_forward
- Be sure to answer all parts. Be sure to include the ion charges. What products are formed when the following amine is treated with HCl: (CH3CH2)2NH arrow draw structure ... + Cl− Be sure to answer all parts. What ammonium salt is formed when the amine is treated with HCl.arrow_forwardWhy is the bond between N and H in amines a polar bond? Hydrogen is more electronegative. B) Nitrogen is more electropositive. C) Nitrogen is more electronegative. D) Hydrogen is electrophilic. E) Nitrogen is electrophilicarrow_forwardAmide hydrolysis in basic conditions forms A. a carboxylic acid and an amine B. a carboxylate salt and an amine 3. an ester and an amine 4. a carboxylic acid and an amine saltarrow_forward
- What is the major Hofmann elimination product formed from each amine?arrow_forwardDefine the simplest method to synthesize an amine ?arrow_forwardQ1. How are the alkaloids classified? Q2. Give any four biological sources of quinine? Give the isolation and extraction of quinine. Q3. Give the synthesis of capsaicin from vanillin.arrow_forward
- Alkylbenzyldimethyl ammonium chloride is a leave-on skin antiseptic used to treat such things as cuts and cold sores. It is also the antiseptic in many hand sanitizers. It is actually a mixture of compounds that differ in the number of carbons (any even number between 8 and 18) in the alkyl group. Show three different sets of reagents (each set composed of an alkyl chloride and an amine) that can be used to synthesize the alkylbenzyldimethyl ammonium chloride shown here.arrow_forwardWhat nitro compound, nitrile, and amide are reduced to each compound?arrow_forward2. Tubulysin D is a potent cytotoxic compound that interferes with mitosis. Identify the following structural features of Tubulysin D: a) Place a box around any amide(s). b) Place a triangle around any aromatic ring(s). c) Place a circle around any carboxylic acid(s).arrow_forward

 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning




