
Concept explainers
a)
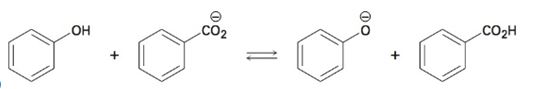
Interpretation:
Using the pKa values the direction in which equilibrium is favored in the reaction given is to be stated.
Concept introduction:
The direction in which equilibrium is favored in the reaction can be ascertained from the pKa values of the substances involved. Lower the pKa value, stronger the acid. Further the relative stabilization by resonance of the conjugate bases produced also has to be considered.
To determine:
The direction in which equilibrium is favored in the reaction using the pKa values given.
b)
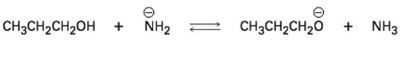
Interpretation:
Using the pKa values the direction in which equilibrium is favored in the reaction given is to be stated.
Concept introduction:
The direction in which equilibrium is favored in the reaction can be ascertained from the pKa values of the substances involved. Lower the pKa value, stronger the acid. Further the relative stabilization by resonance of the conjugate bases produced also has to be considered.
To determine:
The direction in which equilibrium is favored in the reaction using the pKa values.
c)
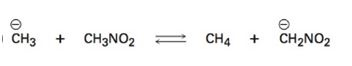
Interpretation:
Using the pKa values the direction in which equilibrium is favored in the reaction given is to be stated.
Concept introduction:
The direction in which equilibrium is favored in the reaction can be ascertained from the pKa values of the substances involved. Lower the pKa value, stronger the acid. Further the relative stabilization by resonance of the conjugate bases produced also has to be considered.
To determine:
The direction in which equilibrium is favored in the reaction using the pKa values.
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Organic Chemistry
- What is the the average Kc (equilibrium constant) based on this table?arrow_forwardHow did [CO32-] decrease while DIC increased? (Ocean Carbon Equilibrium Gizmoarrow_forwardIf the K for a reaction is determined to 3.81 at 20.26 ºC, then what is ΔG for the reaction (in J/mol, round to the nearest one)?arrow_forward
- One of the steps in the production of sulfuric acid involves the catalytic oxidation of sulfur dioxide. 2 SO2 (g) + O2 (g) → 2 SO3 (g) What is the equilibrium expression?arrow_forwardNitrous acid is a weak acid and has a concentration of 0.65 mol/L. What is the pH of the solution when equilibrium is established when Ka=4.5x10-4? b) The value of Kb for nitrous acid is ___________________ c) The percent ionization for nitrous acid is ____________%arrow_forward2IBr(g) ⇌ I2(g) + Br2(g), Kc = 8.54 × 10–3 at 150 °C. If 0.0400 mol of IBr is charged to a 1.00 L container and heated to 150 °C, what is the equilibrium concentration of IBr?arrow_forward
- Consider the endothermic decomposition of carbonic acid below H2CO3(aq) ⇌ CO2(g) + H2O(1) Where would the equilibrium shift if the volume of the system decreases at constant temperature?, and where would the equilibrium shift if N2 gas is introduced in the system?arrow_forwardWhat is the pka of hydrogen (H2)?arrow_forwardConsider the reaction O2 (g) + N2 (g) --> 2NO (g) What is the equilibrium constant for the formation of nitric oxide at 25°C?arrow_forward
- The conversion of oxygen to ozone has a very small equilibrium constant. 3/2 O2(g) O3(g) K = 2.5 1029 (a) What is the value of K when the equation is written using whole-number coefficients? 3 O2(g) 2 O3(g) (b) What is the value of K for the conversion of ozone to oxygen? 2 O3(g) 3 O2(g)arrow_forwardCalculate the equilibrium constant, Kc, for the reaction: 2SO3(g) + 2SO2(g) O2 (g) Given the following data: a) initial conc. of SO3 (g) = 1.32 M b) Equilibrium conc. of SO3 (g) = 0.287 M c) initial conc. of SO2 (g) = 0.282 M d) initial conc. of O2 (g) =0.127 Marrow_forwardCalculate the pH of a 0.20 M HA solution, given the pKa of HA is 2.22.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER




