
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 3, Problem 1CTQ
Interpretation Introduction
Interpretation:Maximum number of electrons that can fit in a single orbital either should be determined.
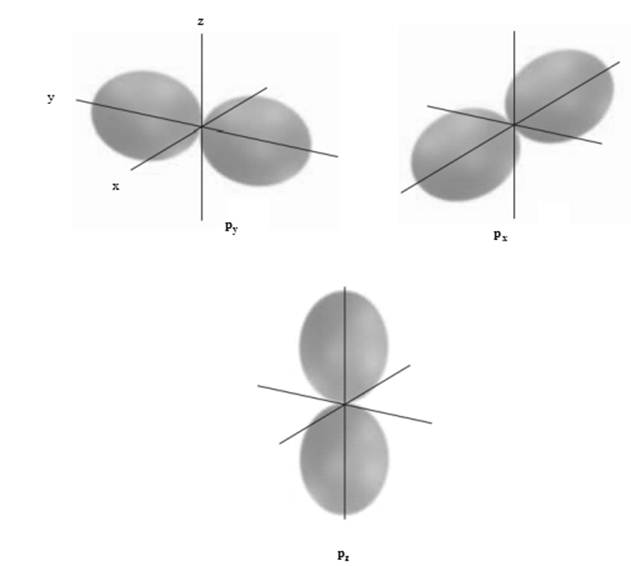
Concept introduction:The shape of three p-orbital is illustrated as follows:
Expert Solution & Answer
Answer to Problem 1CTQ
Two electrons are maximum occupancy of each of the orbitals
Explanation of Solution
The second shell has four orbitals namely
Where,
Inthe second shell, maximum electrons to be accommodated are
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
What atomic terms will be possible for an ns1 np1 electron configuration? and which term is likely to lie lowest in energy?. SHOW FULL AND COMPLETE PROCEDURE IN A CLEAR AND ORDERED WAY
True or false?
The 4d orbital does not exist in the carbon atom.
Justify your answer in 1 sentence or 2.
A principal shell with a value of n=3 would contain s,p,d and f orbits
true or false
Chapter 3 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 3 - Prob. 1CTQCh. 3 - What neutral atom is represented by the electron...Ch. 3 - Prob. 3CTQCh. 3 - Consider any one of the four identical hybrid...Ch. 3 - Prob. 5CTQCh. 3 - Prob. 6CTQCh. 3 - Prob. 7CTQCh. 3 - Prob. 8CTQCh. 3 - Prob. 9CTQCh. 3 - Prob. 10CTQ
Ch. 3 - On the left side of Figure 3.6, label the areas...Ch. 3 - Prob. 12CTQCh. 3 - Prob. 13CTQCh. 3 - Prob. 14CTQCh. 3 - Prob. 15CTQCh. 3 - Now consider the fully formed molecule on the...Ch. 3 - Prob. 1ECh. 3 - Explain why the two molecules below cannot...Ch. 3 - Prob. 3ECh. 3 - Consider the incomplete orbital representation of...Ch. 3 - Consider the following orbital representation of...Ch. 3 - Summarize how one determines the hybridization...Ch. 3 - Explain what is wrong with each of the following...Ch. 3 - Prob. 8ECh. 3 - Prob. 9ECh. 3 - Complete the following tables, and memorize their...Ch. 3 - Draw orbital representations of bonding in water...Ch. 3 - Draw electron configuration diagrams for carbon in...Ch. 3 - Prob. 13E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Is this arrangement of electrons possible, why or why not ? what would happen, eventually, to the electron in the highest energy level in model 1? Write out the ground state electron configurations for this atom.arrow_forwardThe numerator has 4 sf and denominator 2.65 3sf and 4.3 x 10^-2 has 2 sf but this is addition, could explain me this part, I am confusedarrow_forwardFor the configuration why would you leave off the 1s^2 and replace it with [He]?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning