
(a)
Interpretation:
The given carbon oxygen-containing compound has a carbonyl group or not has to be indicated.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom.
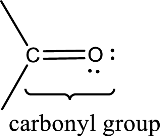
(a)
Answer to Problem 4.1EP
Carbonyl group is present in this compound.
Explanation of Solution
Given compound is,
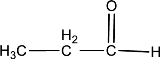
In the above structure, a double bond is present between the carbon and oxygen atom. This is the carbonyl group.
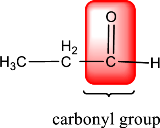
Therefore, the compound that is drawn above has a carbonyl group in its structure.
The given compound contains a carbonyl group in its structure.
(b)
Interpretation:
The given carbon oxygen-containing compound has a carbonyl group or not has to be indicated.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
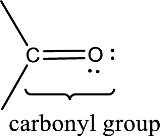
(b)
Answer to Problem 4.1EP
Carbonyl group is not present in this compound.
Explanation of Solution
Given compound is,
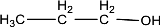
In the above structure, no double bond is present between the carbon and oxygen atom. Therefore, the compound that is given does not have a carbonyl group in its structure.
The given compound does not contain a carbonyl group in its structure.
(c)
Interpretation:
The given carbon oxygen-containing compound has a carbonyl group or not has to be indicated.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
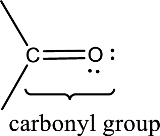
(c)
Answer to Problem 4.1EP
Carbonyl group is not present in this compound.
Explanation of Solution
Given compound is,

In the above structure, no double bond is present between the carbon and oxygen atom. Therefore, the compound that is given does not have a carbonyl group in its structure.
The given compound does not contain a carbonyl group in its structure.
(d)
Interpretation:
The given carbon oxygen-containing compound has a carbonyl group or not has to be indicated.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
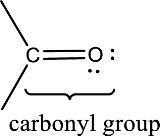
(d)
Answer to Problem 4.1EP
Carbonyl group is present in this compound.
Explanation of Solution
Given compound is,
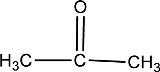
In the above structure, a double bond is present between the carbon and oxygen atom. This is the carbonyl group.
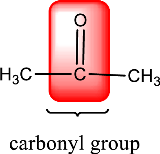
Therefore, the compound that is drawn above has a carbonyl group in its structure.
The given compound contains a carbonyl group in its structure.
Want to see more full solutions like this?
Chapter 4 Solutions
Organic And Biological Chemistry
- What is the functional isomer of butanal? butanoic acid 1-butanol butanone methyl propanoate Other:arrow_forwardGive the IUPAC names of structures containing two carbon atoms for the following classes of compounds: (a) ether: (b) aldehyde: (c) carboxylic acid: (d) ester:arrow_forwardFor embalming purposes, phenol has the properties of preserving, disinfecting, and ___ . cauterizing cut tissues adding a pink glow to the body adding a nice fragrance anticoagulantarrow_forward
- Alcohols contain which functional group? amine thiol amide hydroxylarrow_forwardWrite structural formulas for the following compounds.(a) ethyl isopropyl ether (b) di-n-butyl etherarrow_forwardExplain why alcohols have much higher boiling points than hydrocarbons and alkyl halides of similar molecular weight.arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





