
Interpretation: The method of writing the polyatomic ion in the formula from a chemical name needs to be explained.
Concept Introduction: An ionic compound is composed of cation and anion. Here cation is positively charged and anion carries negative charge.
The cation and anion can be composed of more than one atoms. Such types of ions are called as polyatomic ions.
Answer to Problem 3RQ
The chemical formula of any compound can be written with criss-cross method in which the valency of both cation and anion are cross multiply to get the simplest whole number of elements in the formula.
Explanation of Solution
The chemical formula of any compound can be written with criss-cross method in which the valency of both cation and anion are cross multiply to get the simplest whole number of elements in the formula.
For example; aluminum carbonate is composed of aluminium ion and carbonate ion. Here carbonate ion is polyatomic ion as it consists of one C and three O atoms. Since aluminum has +3 charge and carbonate ion has -2 charge, so to balance the charges, there must be 2 aluminum and 3 carbonate ions in the formula.
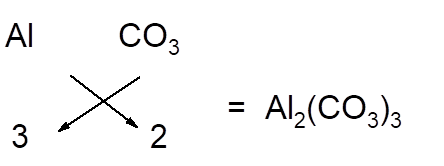
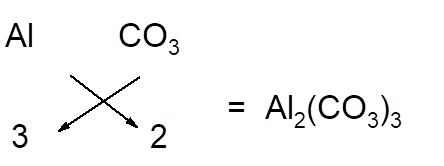
Chapter 4 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





