
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 4.4, Problem 4.6P
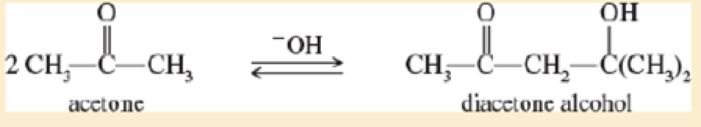
Under base-catalyzed conditions two molecules of acetone can condense to form diacetone alcohol. At room temperature (25 °C), about 5% of the acetone is converted to d acetone alcohol Determine the value of ∆G° for this reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Organic Chemistry (9th Edition)
Ch. 4.3A - Draw Lewis structures for the following free...Ch. 4.3B - a. Write the propagation steps leading to the...Ch. 4.3C - Prob. 4.3PCh. 4.3C - Prob. 4.4PCh. 4.4 - The following reaction has a value of G =...Ch. 4.4 - Under base-catalyzed conditions two molecules of...Ch. 4.5B - When ethene is mixed with hydrogen in the presence...Ch. 4.5B - For each reaction, estimate whether S for the...Ch. 4.7 - a. Propose a mechanism for the free radical...Ch. 4.7 - a. Using bond-dissociation enthalpies from...
Ch. 4.8 - The reaction of tert-butyl chloride with methanol...Ch. 4.8 - Under certain conditions, the bromination of...Ch. 4.8 - When a small piece of plat num is added to a...Ch. 4.10 - Prob. 4.14PCh. 4.10 - Prob. 4.15PCh. 4.12 - The bromination of methane proceeds through the...Ch. 4.12 - a. Using me BDEs in Table4-2 (page 167 ), compute...Ch. 4.13A - What would be the product ratio in the...Ch. 4.13A - Classify each hydrogen atom in the following...Ch. 4.13B - Use the bond-dissociation enthalpies in Tabte4-2...Ch. 4.13B - Prob. 4.21PCh. 4.13B - Prob. 4.22PCh. 4.14 - a. Compute the heats of reaction for abstraction...Ch. 4.14 - 2,3-Dimethylbutane reacts with bromine in the...Ch. 4.14 - Prob. 4.25PCh. 4.15 - Prob. 4.26PCh. 4.15 - Prob. 4.27PCh. 4.16A - Prob. 4.28PCh. 4.16A - Prob. 4.29PCh. 4.16B - Prob. 4.30PCh. 4.16C - Prob. 4.31PCh. 4.16C - Acetonitrile (CH3C N) is deprotonated by very...Ch. 4.16D - Prob. 4.33PCh. 4 - The following reaction is a common synthesis used...Ch. 4 - Consider the following reaction-energy diagram. a....Ch. 4 - Draw a reaction-energy diagram for a one-step...Ch. 4 - Draw a reaction-energy diagram for a two-step...Ch. 4 - Prob. 4.38SPCh. 4 - Treatment of tert-butyl alcohol with concentrated...Ch. 4 - Label each hydrogen atom in the following...Ch. 4 - Prob. 4.41SPCh. 4 - Prob. 4.42SPCh. 4 - Prob. 4.43SPCh. 4 - Prob. 4.44SPCh. 4 - Prob. 4.45SPCh. 4 - Prob. 4.46SPCh. 4 - For each compound, predict the major product of...Ch. 4 - When exactly 1 mole of methane is mixed with...Ch. 4 - Prob. 4.49SPCh. 4 - Prob. 4.50SPCh. 4 - Prob. 4.51SPCh. 4 - When dichloromethane is treated with strong NaOH,...Ch. 4 - Prob. 4.53SPCh. 4 - When a small amount of iodine is added to a...Ch. 4 - Prob. 4.55SPCh. 4 - When healthy, Earths stratosphere contains a low...Ch. 4 - Prob. 4.57SPCh. 4 - lodination of alkanes using iodine (I2) is usually...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- According to Lambert, leaves lying in the yard and playing cards that are in disarray on a table have not undergone an increase in their thermodynamic entropy. Suggest another reason why leaves and playing cards may not be a good analogy for the entropy of a system containing, for example, only H2O molecules or only O2 molecules.arrow_forwardDiethyl ether, (C2H5)2O, was once used as an anesthetic. Calculate the entropy change, rS, for the vaporization of ether if its heat of vaporization is 26.0 kJ/mol at the boiling point of 35.0 C.arrow_forwardWhat is the sign of the standard Gibbs free-energy change at low temperatures and at high temperatures for the synthesis of ammonia? 3H2(g) + N2(g) 2NH3(g)arrow_forward
- At room temperature, the entropy of the halogens increases from I2 to Br2 to Cl2. Explain.arrow_forwardHuman DNA contains almost twice as much information as is needed to code for all the substances produced in the body. Likewise, the digital data sent from Voyager II contained one redundant bit out of every two bits of information. The Hubble space telescope transmits three redundant bits for every bit of information. How is entropy related to the transmission of information? What do you think is accomplished by having so many redundant bits of information in both DNA and the space probes?arrow_forwardTetrachloromethane (carbon tetrachloride), CCl4, has a normal boiling point of 76.7C and an enthalpy of vaporization, Hvap, of 29.82 kJ/mol. Estimate the entropy of vaporization, Svap. Estimate the free energy of vaporization, Gvap, at 25C.arrow_forward
- The standard molar entropy of methanol vapor, CH3OH(g), is 239.8 J K1 mol-1. (a) Calculate the entropy change for the vaporization of 1 mol methanol (use data from Table 16.1 or Appendix J). (b) Calculate the enthalpy of vaporization of methanol, assuming that rS doesnt depend on temperature and taking the boiling point of methanol to be 64.6C.arrow_forwardWhat is entropy? Why is entropy important?arrow_forwardCells use the hydrolysis of adenosine triphosphate, abbreviated as ATP, as a source of energy. Symbolically, this reaction can be written as ATP(aq)+H2O(l)ADP(aq)+H2PO4(aq) where ADP represents adenosine diphosphate. For this reaction, G =30.5 kJ/mol. a. Calculate K at 25C. b. If all the free energy from the metabolism of glucose C6H12O6(s)+6O2(g)6CO2(g)+6H2O(l) goes into forming ATP from ADP, how many ATP molecules can be produced for every molecule of glucose?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY