
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 4.8, Problem 4.13P
When a small piece of plat num is added to a mixture of ethene and hydrogen, the following reaction occurs:
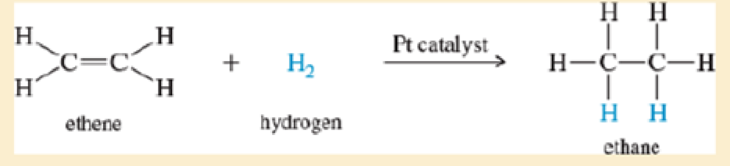
Doubling the concentration of hydrogen has no effect on the reaction rate. Doubling the concentration of ethene also has no effect.
- a. What is the kinetic order of this reaction with respect to ethene? With respect to hydrogen? What is the overall order?
- b. Write the unusual rate equation for this reaction.
- c. Explain this strange rate equation and suggest what one might do to accelerate the reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1) If the reaction order of reactant A is 1, and the reaction order of reactant B is 2, what is the overall reaction order?
2) How is the value of the rate constant determined?
3) How does the value of the rate constant effect the reaction rate?
The rate of a certain reaction is given by the following rate law: rate=kNO2O2
Use this information to answer the questions below.
What is the reaction order in NO?
What is the reaction order in O2?
What is overall reaction order?
At a certain concentration of NO and O2, the initial rate of reaction is 79.0 M / s. What would the initial rate of the reaction be if the concentration of NO were doubled? Round your answer to
3
significant digits.
Ms
The rate of the reaction is measured to be 6.0 x 104 M / s when [NO] = 0.70 M and [O2] = 1.1 M. Calculate the value of the rate constant. Round your answer to
2
significant digits.
=k⋅M−2s−1
The rate of a certain reaction is given by the following rate law:
rate =kNO2O2
Use this information to answer the questions below.
What is the reaction order in NO?
What is the reaction order in O2?
What is overall reaction order?
At a certain concentration of NO and O2, the initial rate of reaction is 79.0 M / s. What would the initial rate of the reaction be if the concentration of NO were doubled? Round your answer to
3
significant digits.
Ms
The rate of the reaction is measured to be 6.0 x 104 M / s when [NO] = 0.70 M and [O2] = 1.1 M. Calculate the value of the rate constant. Round your answer to
2
significant digits.
=k⋅M−2s−1
Chapter 4 Solutions
Organic Chemistry (9th Edition)
Ch. 4.3A - Draw Lewis structures for the following free...Ch. 4.3B - a. Write the propagation steps leading to the...Ch. 4.3C - Prob. 4.3PCh. 4.3C - Prob. 4.4PCh. 4.4 - The following reaction has a value of G =...Ch. 4.4 - Under base-catalyzed conditions two molecules of...Ch. 4.5B - When ethene is mixed with hydrogen in the presence...Ch. 4.5B - For each reaction, estimate whether S for the...Ch. 4.7 - a. Propose a mechanism for the free radical...Ch. 4.7 - a. Using bond-dissociation enthalpies from...
Ch. 4.8 - The reaction of tert-butyl chloride with methanol...Ch. 4.8 - Under certain conditions, the bromination of...Ch. 4.8 - When a small piece of plat num is added to a...Ch. 4.10 - Prob. 4.14PCh. 4.10 - Prob. 4.15PCh. 4.12 - The bromination of methane proceeds through the...Ch. 4.12 - a. Using me BDEs in Table4-2 (page 167 ), compute...Ch. 4.13A - What would be the product ratio in the...Ch. 4.13A - Classify each hydrogen atom in the following...Ch. 4.13B - Use the bond-dissociation enthalpies in Tabte4-2...Ch. 4.13B - Prob. 4.21PCh. 4.13B - Prob. 4.22PCh. 4.14 - a. Compute the heats of reaction for abstraction...Ch. 4.14 - 2,3-Dimethylbutane reacts with bromine in the...Ch. 4.14 - Prob. 4.25PCh. 4.15 - Prob. 4.26PCh. 4.15 - Prob. 4.27PCh. 4.16A - Prob. 4.28PCh. 4.16A - Prob. 4.29PCh. 4.16B - Prob. 4.30PCh. 4.16C - Prob. 4.31PCh. 4.16C - Acetonitrile (CH3C N) is deprotonated by very...Ch. 4.16D - Prob. 4.33PCh. 4 - The following reaction is a common synthesis used...Ch. 4 - Consider the following reaction-energy diagram. a....Ch. 4 - Draw a reaction-energy diagram for a one-step...Ch. 4 - Draw a reaction-energy diagram for a two-step...Ch. 4 - Prob. 4.38SPCh. 4 - Treatment of tert-butyl alcohol with concentrated...Ch. 4 - Label each hydrogen atom in the following...Ch. 4 - Prob. 4.41SPCh. 4 - Prob. 4.42SPCh. 4 - Prob. 4.43SPCh. 4 - Prob. 4.44SPCh. 4 - Prob. 4.45SPCh. 4 - Prob. 4.46SPCh. 4 - For each compound, predict the major product of...Ch. 4 - When exactly 1 mole of methane is mixed with...Ch. 4 - Prob. 4.49SPCh. 4 - Prob. 4.50SPCh. 4 - Prob. 4.51SPCh. 4 - When dichloromethane is treated with strong NaOH,...Ch. 4 - Prob. 4.53SPCh. 4 - When a small amount of iodine is added to a...Ch. 4 - Prob. 4.55SPCh. 4 - When healthy, Earths stratosphere contains a low...Ch. 4 - Prob. 4.57SPCh. 4 - lodination of alkanes using iodine (I2) is usually...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- One experimental procedure that can be used to determine the rate law of a reaction is the method of initial rates. What data are gathered in the method of initial rates, and how are these data manipulated to determine k and the orders of the species in the rate law? Are the units for k. the rate constant, the same for all rate laws? Explain. If a reaction is first order in A, what happens to the rate if [A] is tripled? If the initial rate for a reaction increases by a factor of 16 when [A] is quadrupled, what is the order of n? If a reaction is third order in A and [A] is doubled, what happens to the initial rate? If a reaction is zero order, what effect does [A] have on the initial rate of a reaction?arrow_forward1)Calculate the Reaction Rate for each trial using an average of the Time required for the reaction to go to completion. Add these to your above Table. 2)Calculate the Reaction Order for each Species; m and n.arrow_forwardConsider the following reaction: A + B → products. The rate law was found to be rate = k[A][B]^2. A)If the concentration of A is doubled while the concentration of B is kept constant, how will this affect the rate of the reaction? How will this affect the time required for the reaction to reach completion? B)If the concentration of B is doubled while the concentration of A is kept constant, how will this affect the rate of the reaction? How will this affect the time required for the reaction to reach completion? C)If the concentration of B is doubled while the concentration of A is halved, how will this affect the rate of the reaction? How will this affect the time required for the reaction to reach completion?arrow_forward
- The rate law of a reaction is given below.From the given rate law, what will happen to the reaction time and reaction rate if the concentration of one of the reactants is doubled while keeping everything the same? why?Also, why the starch solution used as an indicator in an experiment turned blue at the end of the reaction?arrow_forward1. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. The equation for the reaction is: Zn (s) + 2HCl (aq) → H2 (g) + ZnCl2 (aq) It is determined that the rate of Zn consumption is -0.00040 mol/s. What would be the rate of HCl consumption Answer to 6 decimal places 2. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. The equation for the reaction is: Zn (s) + 2HCl (aq) → H2 (g) + ZnCl2 (aq) If initially there was 0.00400 moles of Zn and after 17 s there was 0.00160 moles of zinc, what is the rate of reaction in moles of Zn consumed per second? Answer to 6 decimal places 3. For the reaction C6H12 + 9 O2→6 CO2 + 6 H2O, select all the correct relations of rate. Question 3 options: 16∆[CO2]∆t 16∆[H2O]∆t -∆[C6H12]∆t 4. For the reaction 3 H2 + N2 → 2 NH3, what is the rate of reaction when 0.062 moles of NH3 at 6.0 s then becomes 0.190 moles of…arrow_forwardThe rate of a certain reaction is given by the following rate law: rate=kN2H22 Use this information to answer the questions below. What is the reaction order in N2? What is the reaction order in H2? What is overall reaction order? At a certain concentration of N2 and H2, the initial rate of reaction is 57.0 M / s. What would the initial rate of the reaction be if the concentration of N2 were halved? Round your answer to 3 significant digits. Ms The rate of the reaction is measured to be 0.770 M / s when [N2] = 0.73 M and [H2] = 0.65 M. Calculate the value of the rate constant. Round your answer to 2 significant digits. =k⋅M−2s−1arrow_forward
- How are integrated rate laws used to determine reaction order? What is the order for the reactant if a plot of a) ln [reactant] vs. time is linear? b) 1/[reactant] vs. time is linear? c) [reactant] vs. time is linear?arrow_forward2.2 A certain reaction has the rate law: Rate = k [A]. The half-life of this reaction is 20 minutes.a) Calculate the rate constant for this reaction.b) How much time would be required for this reaction to be 60% complete?2.3 What are the two requirements that must be satisfied for reactants to collidesuccessfully (in order to rearrange and form products)?arrow_forward1. base on the data,what how do we find rate constant for this reaction at room temperature? 2. what cause the formation of the purple color ??arrow_forward
- In a clock reaction experiment using iodine, ascorbic acid, hydrogen peroxide, water, and corn starch, a color change is used to determine the reaction rate and reaction time. If the amout of ascorbic acid is increased each run, what do we expect to happen to the rate of reaction and reaction time? Why?arrow_forwardConsider a reactant with an order of "1." What effect does that reactant have on the rate equation when the concentration is doubled? a.) The reaction rate is doubled with respect to that reactant. b.) The reaction rate isn't changed by individual reactants so there will be no change. c.) The reaction rate quadruples with respect to that reactant. d.) The order doesn't effect the reaction so there will be no change..arrow_forwardThis week's experiment is about rate laws. If the initial rate of reaction is 1.92 M/s for an initial concentration of 0.66 M for a first order reaction, what is the rate constant? Report your answer to 3 decimal places.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY