
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 21PP
Practice Problem 5.21
The following are formulas for three compounds, written in noneclipsed conformations. In each instance tell which compound (A, 8, or C on the previous page) each formula represents.
(a)
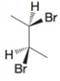
(b)
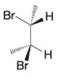
(c)
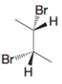
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Take a look at the butane conformers below.
Identify:
(a)Which is an anti conformation in Newman?
(b)Which is a Gauche conformation?
(c)Which is the more stable Sawhorse conformer?
(d)Which has the same potential energy/strain with ALS?
2.35 Consider 1-bromo-2-methylpropane and draw the following.(a) The staggered conformation(s) of lowest energy(b) The staggered conformation(s) of highest energy
2.34 Consider 1-bromopropane, CH3CH2CH2Br.(a) Draw a Newman projection for the conformation in which !CH3 and !Br are anti (dihedral angle 1808).(b) Draw Newman projections for the conformations in which !CH3 and !Br are gauche (dihedral angles 608 and 3008).(c) Which of these is the lowest energy conformation?(d) Which of these conformations, if any, are related by reflection?
Chapter 5 Solutions
Organic Chemistry
Ch. 5 - Prob. 1PPCh. 5 - Prob. 2PPCh. 5 - Prob. 3PPCh. 5 - Prob. 4PPCh. 5 - Prob. 5PPCh. 5 - Prob. 6PPCh. 5 - Prob. 7PPCh. 5 - Practice Problem 5.8 Write three-dimensional...Ch. 5 - Prob. 9PPCh. 5 - Prob. 10PP
Ch. 5 - Practice Problem 5.11 List the substituents in...Ch. 5 - Prob. 12PPCh. 5 - Practice Problem 5.13 Tell whether the two...Ch. 5 - Prob. 14PPCh. 5 - Prob. 15PPCh. 5 - Prob. 16PPCh. 5 - Prob. 17PPCh. 5 - Prob. 18PPCh. 5 - Prob. 19PPCh. 5 - Prob. 20PPCh. 5 - Practice Problem 5.21 The following are formulas...Ch. 5 - Practice Problem 5.22 Write three-dimensional...Ch. 5 - Prob. 23PPCh. 5 - Practice Problem 5.24 Give names chat include (R)...Ch. 5 - Prob. 25PPCh. 5 - Prob. 26PPCh. 5 - Prob. 27PPCh. 5 - Prob. 28PPCh. 5 - Practice Problem 5.29 Write formulas for all of...Ch. 5 - Prob. 30PPCh. 5 - Prob. 31PPCh. 5 - Prob. 32PPCh. 5 - Prob. 33PCh. 5 - Prob. 34PCh. 5 - 5.35 Designate the (R) or (S) configuration at...Ch. 5 - Prob. 36PCh. 5 - (a) Write the structure of...Ch. 5 - Shown below are Newman projection formulas for...Ch. 5 - 5.39 Write appropriate structural formulas...Ch. 5 - Discuss whether each of the following compounds...Ch. 5 - Prob. 42PCh. 5 - Prob. 43PCh. 5 - Compound F has the molecular formula C5H8 and is...Ch. 5 - Prob. 45PCh. 5 - Prob. 46PCh. 5 - Prob. 47PCh. 5 - For the following molecule, draw its enantiomer as...Ch. 5 - 5.49 (Use models to solve this...Ch. 5 - 5.50 (Use models to solve this...Ch. 5 - (Use models co solve this problem.) Write...Ch. 5 - 5.52 Tartaric acid was an important compound in...Ch. 5 - Prob. 53PCh. 5 - Prob. 54PCh. 5 - Prob. 55PCh. 5 - Prob. 1LGPCh. 5 - Prob. 2LGPCh. 5 - Prob. 3LGPCh. 5 - Prob. 2QCh. 5 - Prob. 3QCh. 5 - Prob. 4QCh. 5 - Select the words that best describe what happens...Ch. 5 - Prob. 6QCh. 5 - Prob. 7QCh. 5 - Prob. 8QCh. 5 - 5.9 Which is untrue about the following...Ch. 5 - Prob. 10Q
Additional Science Textbook Solutions
Find more solutions based on key concepts
For the generic equilibrium HA(aq) ⇌ H + (aq) + A- (aq), which of these statements is true?
The equilibrium con...
Chemistry: The Central Science (13th Edition)
For Practice 1.1
Is each change physical or chemical? Which kind of property (chemical or physical) is demonst...
Principles of Chemistry: A Molecular Approach (3rd Edition)
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
Q3. Which change is a physical change?
a) Wood burning
b) Iron rusting
c) Dynamite exploding
d) Gasoline ev...
Chemistry: A Molecular Approach (4th Edition)
Ortho-phosphoric acid (H3PO4) is produced as a dilute aqueous solution that must be concentrated before further...
Elementary Principles of Chemical Processes, Binder Ready Version
1.3 Obtain a bottle of multivitamins and read the list of ingredients. What are four chemicals from the list?
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the two chair conformations of the following substituted cyclohexanes. In each case, label the more stable conformation.(a) trans-1,2-diethylcyclohexanearrow_forward(a) which if the structure of trans-1,2-dimethylcyclopentane? (b) which is the most stable conformation of 1-bromo-2-ethylcyclohexane? (c) which is the least stable conformation of 1-bromo-2-ethylcyclohexane? (d) which is the more stable configuration of 1,3-dimethylcyclopentane? *Et = ethylarrow_forwardConsider 1-bromopropane, CH3CH2CH2Br. (a) Draw a Newman projection for the conformation in which CH3 and -Br are anti (dihedral angle 180°). (b) Draw Newman projections for the conformations in which - CH3 and -Br are gauche (dihedral angles 60° and 300°). (c) Which of these is the lowest energy conformation? (d) Which of these conformations, if any, are related by reflection?arrow_forward
- 2.35 Write a structural formula for each of the following compounds: (a) 6-Isopropyl-2,3-dimethylnonane (b) 4-tert-Butyl-3-methylheptane (c) 4-Isobutyl-1,1-dimethylcyclohexane (d) sec-Butylcycloheptane (e) Cyclobutylcyclopentanearrow_forward3. (20) Cyclohexane may go through ring-flipping as shown below. 5 (a) Draw both of the possible chair conformations for cis-1-tert-butyl-4-methylcyclohexane by copying the scheme above followed by adding appropriate substituents to axial or equatorial positions. (b) Circle the more stable conforms of (A) and (B)arrow_forward5.76 2,3-Dibromoprop-1-ene (C3H4Br₂) has four H atoms. Suppose that any of these H atoms can be replaced by a Cl atom to yield a molecule with the formula C3H3Br₂Cl. (a) Identify two H atoms where this substitution would yield constitutional isomers of C3H3Br₂Cl; (b) enantiomers of C3H3Br₂Cl; (c) diastereomers of C3H3Br₂Cl. co 11 HHH Juods H Brugtrio 2 bas A 2,3-Dil so ontemme A chon Br 2,3-Dibromoprop-1-enearrow_forward
- (a) All Isomers have the same molecular formulae. Explain this further. Hint what does it mean to have the same molecular formulae? (b) What is Constitutional (structural) isomerism? Hint, isomers are different, so what is different between a pair of constitutional isomers (see the chart on the last page for examples). (c) What is Conformational isomerism?arrow_forwardFor each of the following molecular formulas, determine the number of elements of unsaturation,and draw three examples.(a) C4H4Cl2 (b) C4H8Oarrow_forward3B. For each compound below, draw two chair conformations. Indicate whether the substituents are axial or equatorial. Indicate which chair conformation is more stable. (you need to draw conformations of the exact molecule, not its mirror image or diastereomer) (a) (b) (c) Ме Me Et "Et Et Mearrow_forward
- Draw the more stable chair conformation for each of the following disubstituted cyclohexanes. (a) OH (b) OH (c) (d) (e) (f) „CBr3 CBr3 "Cl3 CCI3 Cl3arrow_forwardConsider 1-bromopropane, CH3CH2CH2Br. (a) Which of these is the lowest energy conformation?arrow_forwardDraw the alternative chair conformations for the cis and trans isomers of 1,2-dimethylcyclohexane, 1,3- dimethylcyclohexane, and 1,4-dimethylcyclohexane. (a ) Indicate by a label whether each methyl group is axial or equatorial. (b) For which isomer(s) are the alternative chair conformations of equal stability? (c) For which isomer(s) is one chair conformation more stable than the other?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License