
Concept explainers
(a)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
Molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image. But not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are superimposable.
Explanation of Solution
Molecules in the given pair are
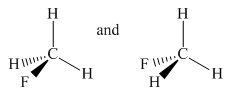
In the above pair, both molecules have carbon as the central atom with one fluorine atom and three hydrogen atoms as an attachment. In the left molecule, fluorine is pointing towards the observer and one hydrogen atom is pointing away from the observer while in the second molecule fluorine is pointing away from the observer and one hydrogen atom is pointing towards the observer.
To check the molecule in different orientation the molecule present at right is rotated.
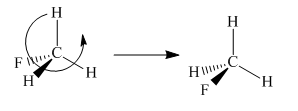
The molecule after the rotation has the exact similar orientation of the atoms as the left molecule in the given pair i.e. all atoms are lined up perfectly.
Thus both the molecules in the given pair are superimposable.
All atoms of both molecules are lined perfectly hence superimposable.
(b)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
Molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image, but not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are superimposable.
Explanation of Solution
Molecules in the given pair are
In given pair, both molecules are mirror images of each other. If the molecule at right is rotated, it gives the molecule exactly similar to the left one.
Thus both the molecules in the given pair are superimposable.
All atoms of both the molecules are lined perfectly hence superimposable.
(c)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
Molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image, but not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are nonsuperimposable.
Explanation of Solution
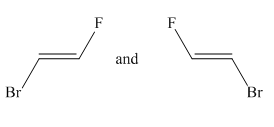
Molecules in the given pair are:
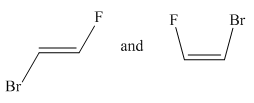
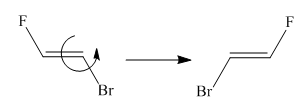
Two molecules given in the pair are the cis-trans isomers of the same molecule. To get the trans isomer from cis, rotation is needed around the double bond which is restricted. Hence the atoms in both molecules are not lined up perfectly, thus the given two molecules are nonsuperimposable.
All atoms of both molecules are not lined perfectly hence nonsuperimposable.
(d)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
The molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image, but not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are superimposable.
Explanation of Solution
Molecules in the given pair are:
![]()
Two molecules given in pairs are the mirror images of each other. If one of the two given molecules is rotated around the central carbon atom it gives the exact similar molecule as another.
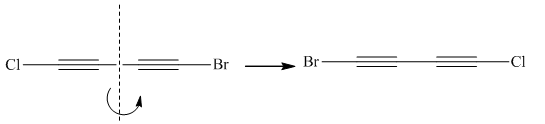
Rotation of the molecule gives the molecule with a similar orientation as another molecule, thus two molecules are superimposable.
All atoms of both molecules are lined perfectly, hence superimposable.
(e)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
The molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image, but not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are superimposable.
Explanation of Solution
Molecules in the given pair are:
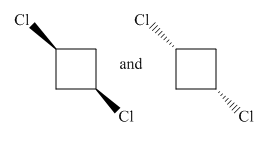
Both molecules in the given pair are isomers of the same molecules thus have the same attachments of the molecule with different orientations. Two chlorine atoms in the left molecule are pointing towards the observer while two chlorine atoms in the right molecule are pointing away from the observer. If one of the molecule rotates with
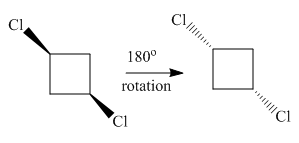
The molecule after the rotation of the left molecule has the exact similar orientation as the molecule at right, all atoms of both molecules are lined perfectly representing the molecules in the given pair are superimposable.
All atoms of both molecules are lined perfectly hence superimposable.
(f)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
Molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image, but not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are nonsuperimposable.
Explanation of Solution
Molecules in the given pair are
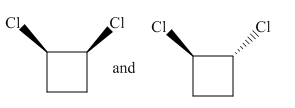
In the molecule at the left, both chlorine atoms are pointing towards the observer while in the molecule at right one chlorine atom is pointing towards the observer and one is pointing away from the observer. To get a similar orientation, rotation of the atom with the ring is needed which is restricted. Thus there is no orientation of both molecules lined perfectly, representing the molecules in the given pair are nonsuperimposable.
All atoms of both molecules are not lined perfectly, hence nonsuperimposable.
(g)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
Molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image. But not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are superimposable.
Explanation of Solution
Molecules in the given pair are
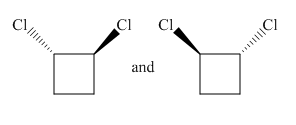
In the given pair both molecules are mirror images of each other having one chlorine atom pointing towards the observer and another pointing away from the observer.
The complete rotation of
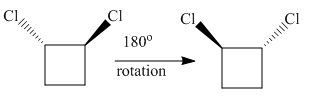
The molecule after the rotation of the left molecule has the exact similar orientation as the molecule at right, all atoms of both molecules are lined perfectly representing the molecules in the given pair are superimposable.
All atoms of both molecules are lined perfectly hence superimposable.
(h)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
The molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image. But not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are nonsuperimposable.
Explanation of Solution
Molecules in the given pair are
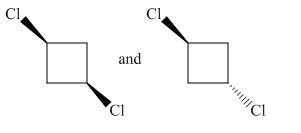
In the molecule at left both chlorine atoms are pointing towards the observer while in the molecule at right one chlorine atom is pointing towards the observer and one is pointing away from the observer. To get a similar orientation, rotation of the atom with the ring is needed which is restricted. Thus no orientation of both molecules lined perfectly, representing the molecules in the given pair are nonsuperimposable.
All atoms of both molecules are not lined perfectly hence nonsuperimposable.
Want to see more full solutions like this?
Chapter 5 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Kindly explain why COSe is the answer in detail.arrow_forwardWhat is the hybridizarion of the atom labeled with arrow in each of the following molecules?arrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Note: This is the last question of my homework. Please take all of the time you need!arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
