
Concept explainers
(a)
Interpretation:
Whether the compounds in the given pair have the same or different boiling points is to be determined.
Concept introduction:
Isomers are the pair of compounds that have the same formula.
Constitutional isomers are the isomers having the same molecular formula but different connectivity. Constitutional isomers must have different physical and chemical properties.
The configurational isomers are not interconvertible by rotating around a single bond.
Enantiomers are configurational isomers having the same connectivity but cannot be interconvertible by rotation around a single bond. The mirror images of enantiomers are non-superimposable. Since enantiomers have the same connectivity of atoms, they should behave identically.
Diastereomers are configurational isomers having same connectivity but are not mirror images of each other. Diastereomers show different physical and chemical properties. Cis-trans isomers are diastereomers.
The boiling point of isomers comes under physical property.
Answer to Problem 5.53P
The given pair of isomers have different boiling points as they are diastereomers of each other.
Explanation of Solution
The given pair of compounds is
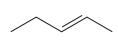
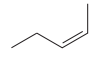
Both the molecules in the given pair have the same molecular formula; they also have the same connectivity. Thus, they are configurational isomers. The given pair of molecules is cis-trans isomers. So, these compounds are diastereomers of each other. Diastereomers show different physical and chemical properties. Thus, the compounds in the given pair should have different boiling points.
The given pair of isomers have different boiling point as they are diastereomers of each other.
(b)
Interpretation:
Whether the compounds in the given pair have the same or different boiling points is to be determined.
Concept introduction:
Isomers are the pair of compounds that have the same formula.
Constitutional isomers are the isomers having the same molecular formula but different connectivity. Constitutional isomers must have different physical and chemical properties.
The configurational isomers are not interconvertible by rotating around a single bond.
Enantiomers are configurational isomers having the same connectivity but cannot be interconvertible by rotation around a single bond. The mirror images of enantiomers are non-superimposable. Since enantiomers have the same connectivity of atoms, they should behave identically.
Diastereomers are configurational isomers that have the same connectivity but are not mirror images of each other. Diastereomers have different physical and chemical properties. Cis-trans isomers are diastereomers of each other.
The boiling point of isomers comes under physical property.
Answer to Problem 5.53P
The given pair of isomers have different boiling points as they are constitutional isomers of each other.
Explanation of Solution
The given pair of compounds is
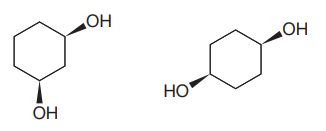
Both the molecules in the given pair have the same molecular formula, so they are isomers.
However, they do not have same connectivity of atoms. In both the compounds, there are two
In the first compound, the
The given pair of isomers have different boiling points as they are constitutional isomers of each other.
(c)
Interpretation:
Whether the compounds in the given pair have the same or different boiling points is to be determined.
Concept introduction:
Isomers are the pair of compounds that have the same formula.
Constitutional isomers are the isomers having the same molecular formula but different connectivity. Constitutional isomers must have different physical and chemical properties.
The configurational isomers are not interconvertible by rotating around a single bond.
Enantiomers are configurational isomers having the same connectivity but cannot be interconvertible by rotation around a single bond. The mirror images of enantiomers are non-superimposable. Since enantiomers have the same connectivity of atoms, they should behave identically.
Diastereomers are configurational isomers that have the same connectivity but are not mirror images of each other. Diastereomers have different physical and chemical properties. Cis-trans isomers are diastereomers of each other.
The boiling point of isomers comes under physical property.
In a Fischer projection, exchanging two groups on an asymmetric carbon atoms gives the opposite stereochemical configuration.
Answer to Problem 5.53P
The given pair of isomers should have different boiling points as they are diastereomers of each other.
Explanation of Solution
The given pair of compounds is
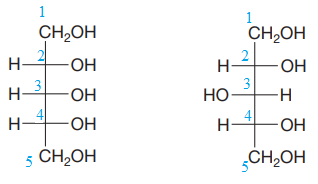
Both the molecules in the given pair have the same molecular formula. They also have the same connectivity. Thus, they are configurational isomers.
Each molecule has three chiral centers at C2, C3, and C4 carbon atoms. Note that in the second compound, the two groups on C3 chiral carbon have been exchanged. This suggests that the stereochemical configurations at C3 carbon atoms in both the compounds are opposite to each other. Remaining stereo centers have the same stereochemical configuration, suggesting that the two compounds are diastereomers of each other. Diastereomers have different physical and chemical properties. Thus, the compounds in the given pair should have different boiling points.
The given pair of isomers have different boiling points as they are diastereomers of each other.
(d)
Interpretation:
Whether the compounds in the given pair have the same or different boiling points is to be determined.
Concept introduction:
Isomers are the pair of compounds that have the same formula.
Constitutional isomers are the isomers having the same molecular formula but different connectivity. Constitutional isomers must have different physical and chemical properties.
The configurational isomers are not interconvertible by rotating around a single bond.
Enantiomers are configurational isomers having the same connectivity but cannot be interconvertible by rotation around a single bond. The mirror images of enantiomers are non-superimposable. Since enantiomers have the same connectivity of atoms, they should behave identically.
Diastereomers are configurational isomers that have the same connectivity but are not mirror images of each other. Diastereomers have different physical and chemical properties. Cis-trans isomers are diastereomers of each other. Cyclic
The boiling point of isomers comes under physical property.
Answer to Problem 5.53P
The given pair of isomers should have different boiling points as they are diastereomers of each other.
Explanation of Solution
The given pair of compounds is
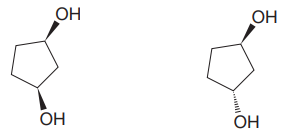
In both the compounds, the
The given pair of isomers have different boiling points as they are diastereomers of each other.
(e)
Interpretation:
Whether the compounds in the given pair have the same or different boiling points is to be determined.
Concept introduction:
Isomers are the pair of compounds that have the same formula.
Constitutional isomers are the isomers having the same molecular formula but different connectivity. Constitutional isomers must have different physical and chemical properties.
The configurational isomers are not interconvertible by rotating around a single bond.
Enantiomers are configurational isomers having the same connectivity but cannot be interconvertible by rotation around a single bond. The mirror images of enantiomers are non-superimposable. Since enantiomers have the same connectivity of atoms, they should behave identically.
The boiling point of isomers comes under physical property.
Answer to Problem 5.53P
The given pair of isomers should have the same boiling point as they are enantiomers of each other.
Explanation of Solution
The given pair of compounds is
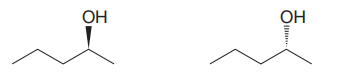
In both the compounds, the
The stereochemical configuration at the chiral center in the first molecule is S as the top-three priority groups are arranged in a counterclockwise manner, and the fourth-priority group is on a dash bond.
The stereochemical configuration at the chiral center in the second molecule is R as the top-three priority groups are arranged in a counterclockwise manner, but the fourth-priority group is on a wedge bond.
Thus, the stereochemical configuration at the chiral centers for two molecules is opposite. This indicates that the two compounds must be enantiomers of each other. Enantiomers have precisely the same physical and chemical properties. Hence the two compounds in the given pair have the same boiling points.
The given pair of isomers should have the same boiling point as they are enantiomers of each other.
Want to see more full solutions like this?
Chapter 5 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Complete the given table below. What properties do each of the following R groups have?arrow_forwardOnly typed explanation otherwise leave it Which of these compounds would have the highest boiling point?arrow_forwarddraw the two chair conformers for the following structures. If one is lower energy, circle the one of each pair .arrow_forward
- Which member in each of the following pairs would have a higher boiling point ?Explain.arrow_forwardBranching of the hydrocarbon chain lowers the boiling points of the lower homologous series, such as the hydrocarbons and alcohols. Therefore, an assumption can be made that branching lowers the ______ forces attraction in these molecules. please answer the blankarrow_forwardDo all please What is the steric number for the three indicated carbon atoms (C1, C2, and C3) in the following molecule?arrow_forward
- need help. Out of the four isomers shown above (menthol, isomenthol, neomenthol, and neoisomenthol), which isomer do you predict is the most stable? It may help to draw out each isomer in its most stable chair conformer to determine which has the most equatorial groups.arrow_forwardDraw a Newman projection for two more staggered conformations of this molecule. Which of your conformations is most stable? Assume that -OH and -CH3 are comparable in size.arrow_forwardHow much more stable is the most stable staggered conformer than the least stable eclipsed conformer?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
