
Concept explainers
(a)
Interpretation:
Fischer projection of the given molecule is to be drawn.
Concept introduction:
A molecule containing a chain of carbon atoms is frequently represented in its zigzag conformation. If it contains multiple asymmetric centers, it is more convenient to draw its Fisher projection. Fischer projections are generally drawn with the longest carbon chain vertical. The two groups attached to each carbon except the first and last are shown on horizontal bonds. The carbon atoms in the middle of the chain are represented by the intersections of vertical and horizontal lines. The vertical lines represent bonds that are oriented away from the viewer while the horizontal bonds are oriented toward the viewer. The most oxidized group must be present at the top of the vertical line.
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the same side of the plane and are shown by wedge bonds, then those groups will be on the opposite sides of the vertical chain in the Fischer projection.
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the opposite sides of the plane and are shown by a wedge and a dash bond, then, in the Fischer projection, those groups will be on the same side of the vertical chain.
In the zig-zag conformation, if two groups on alternate carbon atoms are on opposite sides of the plane and are shown by a wedge and a dash bond, then, in the Fischer projection, those groups will be on the opposite sides of the vertical chain.
Answer to Problem 5.52P
The Fischer projection of the given molecule is
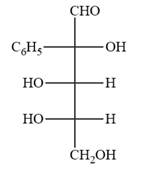
Explanation of Solution
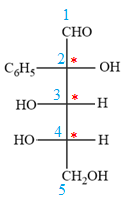
The given molecule is
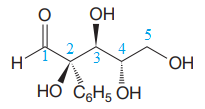
The given molecule consists of a five carbon chain, each of which is numbered. C2, C3, and C4 are the asymmetric carbon atoms. We begin to draw the Fischer projection with the framework shown below:
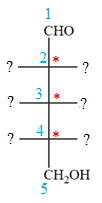
The asymmetric centers are denoted by asterisks. Two of the bonds on each asymmetric center have been left with question marks because two substituents must still be added to each so that the stereochemical configuration at those carbon atoms in the Fischer projection matches with what was given in the dash-wedge notation.
Notice that in the zig-zag conformation, the OH groups on C2 and C3 carbon atoms lie on the same side of the plane and are shown by a wedge bond. Thus, these two groups must lie on opposite sides in the Fischer projection, as shown below:
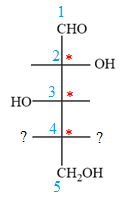
The OH groups on C3 and C4 carbon atoms lie on the opposite side of the plane and are shown by a wedge and a dash bond. Thus, these two groups must lie on the same side in the Fischer projection, as shown below:
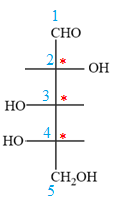
The OH groups on C2 and C4 carbon atoms lie on the opposite side of the plane and are shown by a wedge and a dash bond. Thus, these two groups must lie on the opposite side in the Fischer projection.
Thus, the OH groups on C3 and C4 carbon atoms must lie on the same side of the vertical chain, and OH group on C2 must lie on the opposite side. Then fill in the remaining groups on C2, C3, and C4 carbon atoms to complete the structure as below:
Thus, the structure above is the correct conversion from a given zig-zag conformation to a Fischer projection.
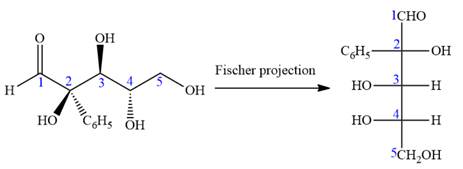
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the opposite sides of the plane and are shown by a wedge and dash bond, then those groups will be on the same side of the vertical chain in the Fischer projection. In the zig-zag conformation, if two groups on adjacent carbon atoms are on opposite sides of the plane and are shown by a wedge and dash bond, then those groups will be on the same side of the vertical chain in the Fischer projection.
Interpretation:
(b)
Fischer projection of the given molecule is to be drawn.
Concept introduction:
A molecule containing a chain of carbon atoms is frequently represented in its zigzag conformation. If it contains multiple asymmetric centers, it is more convenient to draw its Fisher projection. Fischer projections are generally drawn with the longest carbon chain vertical. The two groups attached to each carbon except the first and last are shown on horizontal bonds. The carbon atoms in the middle of the chain are represented by the intersections of vertical and horizontal lines. The vertical lines represent bonds that are oriented away from the viewer while the horizontal bonds are oriented toward the viewer. The most oxidized group must be present at the top of the vertical line.
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the same side of the plane and are shown by wedge bonds, then those groups will be on the opposite sides of the vertical chain in the Fischer projection.
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the opposite sides of the plane and are shown by a wedge and a dash bond, then, in the Fischer projection, those groups will be on the same side of the vertical chain.
In the zig-zag conformation, if two groups on alternate carbon atoms are on opposite sides of the plane and are shown by a wedge and a dash bond, then, in the Fischer projection, those groups will be on the opposite sides of the vertical chain.
Answer to Problem 5.52P
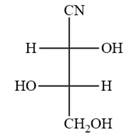
The Fischer projection of the given molecule is
Explanation of Solution
The given molecule is
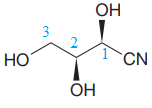
The given molecule consists of a three carbon chain, each of which is numbered. C1 and C2 carbon atoms are the asymmetric carbon atoms. We begin to draw the Fischer projection with the framework shown below:
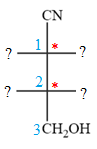
The asymmetric centers are denoted by asterisks. Two of the bonds on each asymmetric center have been left with question marks because two substituents must still be added to each so that the stereochemical configuration at those carbon atoms in the Fischer projection matches with what was given in the dash-wedge notation.
Notice that in the zig-zag conformation, the OH groups on C1 and C2 carbon atoms lie on the same side of the plane and are shown by a wedge bond. Thus, these two groups must lie on opposite side in the Fischer projection, as shown below:
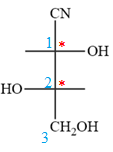
Then fill in the remaining groups on C1 and C2 carbon atoms to complete the structure as below:
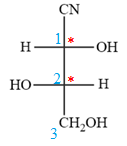
Thus, the structure above is the correct conversion from a given zig-zag conformation to a Fischer projection.
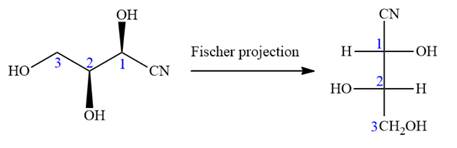
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the opposite sides of the plane and are shown by a wedge and dash bond, then those groups will be on the same side of the vertical chain in the Fischer projection. In the zig-zag conformation, if two groups on adjacent carbon atoms are on opposite sides of the plane and are shown by a wedge and dash bond, then those groups will be on the same side of the vertical chain in the Fischer projection.
Interpretation:
(c)
Fischer projection of the given molecule is to be drawn.
Concept introduction:
A molecule containing a chain of carbon atoms is frequently represented in its zigzag conformation. If it contains multiple asymmetric centers, it is more convenient to draw its Fisher projection. Fischer projections are generally drawn with the longest carbon chain vertical. The two groups attached to each carbon except the first and last are shown on horizontal bonds. The carbon atoms in the middle of the chain are represented by the intersections of vertical and horizontal lines. The vertical lines represent bonds that are oriented away from the viewer while the horizontal bonds are oriented toward the viewer. The most oxidized group must be present at the top of the vertical line.
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the same side of the plane and are shown by wedge bonds, then those groups will be on the opposite sides of the vertical chain in the Fischer projection.
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the opposite sides of the plane and are shown by a wedge and a dash bond, then, in the Fischer projection, those groups will be on the same side of the vertical chain.
In the zig-zag conformation, if two groups on alternate carbon atoms are on opposite sides of the plane and are shown by a wedge and a dash bond, then, in the Fischer projection, those groups will be on the opposite sides of the vertical chain.
Answer to Problem 5.52P
The Fischer projection of the given molecule is
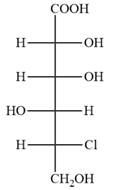
Explanation of Solution
The given molecule is
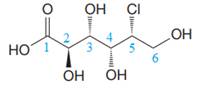
The given molecule consists of a six carbon chain, each of which is numbered. C2, C3, C4, and C5 carbon atoms are the asymmetric carbon atoms. We begin to draw the Fischer projection with the framework shown below:

The asymmetric centers are denoted by asterisks. Two of the bonds on each asymmetric center have been left with question marks because two substituents must still be added to each so that the stereochemical configuration at those carbon atoms in the Fischer projection matches with what was given in the dash-wedge notation.
Notice that in the zig-zag conformation, the OH groups on C2 and C3 carbon atoms lie on the opposite side of the plane and are shown by a wedge and a dash bond. Thus, these two groups must lie on same side in the Fischer projection, as shown below:
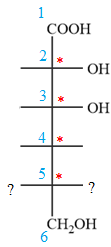
Notice that in the zig-zag conformation, the OH groups on C3 and C4 carbon atoms lie on the same side of the plane and are shown by dash bonds. Thus, these two groups must lie on the opposite sides of the vertical chain in the Fischer projection, as shown below:
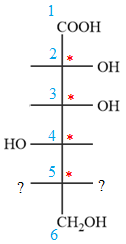
In the zig-zag conformation, the OH group and chlorine atom on C4 and C5 carbon atoms lie on the same side of the plane and are shown by dash bonds. Thus, these two groups must lie on the opposite sides of the vertical chain in the Fischer projection, as shown below:
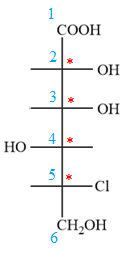
Then place the remaining groups on C1 and C2 carbon atoms to complete the structure as below:
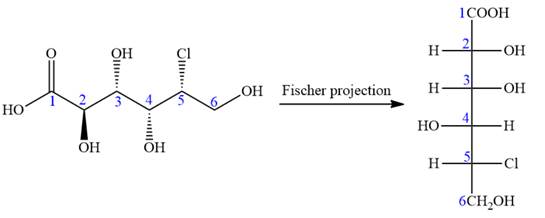
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the opposite sides of the plane and are shown by a wedge and dash bond, then those groups will be on the same side of the vertical chain in the Fischer projection. In the zig-zag conformation, if two groups on adjacent carbon atoms are on opposite sides of the plane and are shown by a wedge and dash bond, then those groups will be on the same side of the vertical chain in the Fischer projection.
Want to see more full solutions like this?
Chapter 5 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- How do you assign the chiral compounds for this chair conformation?arrow_forwardDraw the more stable chair conformation of each of the following molecules.arrow_forwarda) Draw the following compound in topological structure (zig-zag) and name the conformation that is drawn: b) Determine if each of the following molecules is an enantiomer, a diastereoisomer, an isomer in constitution or identical to the molecule in (a) and which is drawn in the box. Solve all parts otherwise I will downvotearrow_forward
- Draw all possible stereoisomers for the molecule in Fischer projection and label the relationship between each of the isomer.arrow_forwarddraw each of the chair configurations of myo inositol. predict which conformation will be more stable, explaining whyarrow_forwardGlucose, when in your bloodstream, conforms to a chair conformation. Using the templates in the picture, draw the two chair conformations of glucose and circle the more stable one.arrow_forward
- Draw the two possible chair conformations for the below molecule. Clearly indicate whether the substituents are axial (a) or equatorial (e).arrow_forwardDraw the most stable chair conformation for the following compounds:arrow_forwardFor the compounds below, draw both chair conformations.arrow_forward
- Which structure below shows the chair conformation of β-D-glucopyranose? The Fischer projection of this compound is shown in the box. Click on a letter A through D to answer.arrow_forwardprovide chari conformations and chair flip, which one is most stable and why?arrow_forwardDraw the two chair conformations of menthol, and identify the more stable conformationarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





